Introduction
In the past decade, transgenic plant expression systems have emerged as a serious competitive force in the large-scale production of recombinant proteins. The first plant-derived heterologous proteins have already reached the market1,2 and detailed economic evaluations have demonstrated their competitiveness against established market sectors3,4. Several plant-derived recombinant therapeutic proteins which are in the final stage of clinical trials include human blood products, vaccine, antibody and growth hormones5. As with a number of products coming to the market, molecular farming in plants is finally coming of age. There have been technological developments on many levels, including transformation methods, control of gene expression, protein targeting and accumulation, the use of different crops as production platforms6 and modifications to alter the structural and functional properties of the product. One of the most driving factors has been yield improvements, as product yield has a significant impact on economic feasibility. To increase the final yield, a number of approaches can be used. One of them is to use a tissue-specific promoter, which can concentrate the protein of interest into targeted tissue and prove advantageous during downstream purification7.
In the present report, a protocol for expression of therapeutic proteins in potato tuber by using patatin, a tuber-specific promoter, was made. Indian tetraploid potato variety, Kufri Bahar was used for the expression of the therapeutic protein, as it is widely grown in Gujarat and other region of India. Potato is a vegetative propagated plant, which minimizes the spread of transgene contamination through pollen, and high tuber biomass makes it suitable for bulk production8.
Erythropoietin (EPO) was chosen as a protein of interest for tuber-specific expression, and to evaluate the capability of performing complex glycosylation in the potato plant. Erythropoietin is a highly glycosylated protein and glycosylation is necessary for its in vivo activity9. Presently, recombinant erythropoietin available on the market is produced from mammalian cells. Mammalian systems have disadvantages in term of cost, scalability and safety10,11. Recombinant human erythropoietin is widely used to treat anemia associated with chronic renal failure, rheumatoid arthritis, acquired immune deficiency syndrome (AIDS), and malignancies, as well as other types of anemia12.
Recombinant EPO was expressed in tobacco BY2 cells. However, the expression level was very low (0.0026%) and expressed protein remained active only in vitro13. Thus, to increase the expression, researchers tried to express this protein in a whole plant using constitutive promoter (tobacco and Arabidopsis) where the side effect of over expression was observed14. Overexpression of EPO protein resulted in stunted vegetative growth, late flowering and male-sterility15.
While in the present project we had chosen to express EPO protein in a tissue-specific manner, by using a tissue-specific approach, we aimed to decrease the metabolic burden to the whole plant and concentrate the protein in targeted tissue, where it can be easily purified. In the present study, potato tubers were selected as an organ of choice for the production of EPO since the same standard processes used in the starch industry may be adapted with little modifications to separate proteins. Also, the tubers, as most of the storage organs, offer a low hydrolytic profile, which facilitate protein stability.
Several genes have been expressed in potato tubers using different techniques with varying degrees of success15–17. Considerable success has been achieved with the patatin promoter, which confers tuber specificity16,18. Most of the reports which use potato tuber as an expresser of therapeutic proteins had generally used the diploid potato variety19–23. However, we aim to use the Indian tetraploid potato variety Kufri Bahar, which is widely grown in Gujarat and other parts of India24.
Materials and Methods
Plasmids, bacterial strains and plants
In the present report, plasmids, bacterial strains and plants were used, as described in Table 1.
Table 1. Plasmids, bacterial strains and plant variety used in this study.
|
Plasmids/Bacterial strains
|
Properties/Purpose
|
Source
|
|---|
pBinB33
pCAMBIA 1281Z
pIRsepo | Contain Patatin Promoter
Used as backbone vector
Contain cDNA of EPO gene | Gifted Dr. Frederik Bornke, Germany
Procured from CAMBIA, Australia
Gifted by Dr. Kirill Alexandrov, Germany |
E. coli DH5α
E. coli HB101
Agrobacterium tumefaciens LBA 4404 | Use for cloning and amplification all plasmids
Used as helper Plasmid in triparental conjugation
Disarmed strains | B.V. Patel PERD centre’s Depository
Procured from DSMZ, Germany
Procured from Netherland culture collection (NCCB), Netherland |
Plant Variety
Solanum tuberosum (Kufri Bahar)
| Used as an expression host for EPO gene | Gifted by Dr. N.H. Patel (Potato Research station, DISA) |
Growth and maintenance of bacterial cultures
Escherichia coli DH5α and Agrobacterium LBA4404 cultures were grown in luria broth (LB) medium. E. coli cultures were grown at 37°C while Agrobacterium cultures were grown at 28°C at 175 rpm. When necessary, antibiotics were added for E. coli (chloramphenicol [Sigma] 35mg/ml) and for Agrobacterium (chloramphenicol 35 mg/ml and rifampicin [Hi-media] 50mg/ml).
Construction of plant rEPO expression vectors
All enzymes which were used for cloning where purchased from MBI fermentas. Tuber-specific promoter patatin (B33) was excised from the vector pBinB33 using Eco RI and Bam HI Restriction Endonuclease (RE) sites and cloned into binary vector pCAMBIA1281Z using the same RE sites. pCAMBIA1281Z contained GUS as a reporter protein. The patatin gene was cloned upstream to the GUS gene at Eco RI and Bam HI RE sites, resulting in a plasmid named pPERDB33 (Figure 1a). For the expression of the EPO gene, the cDNA sequence of EPO was excised from the pIRsepo and cloned in to pPERDB33 in place of the GUS gene by using Nco I and Bst EII RE sites (Figure 1b). The resulting plasmid was named pPERDB33cEPO, which has the EPO gene downstream to the patatin promoter (Figure 1b). pPERDB33cEPO was then introduced in to E. coli DH5α for maintenance and further experimentation. pPERDB33cEPO was also introduced into Agrobacterium tumefaciens strain LBA 4404 by using triparental mating25.
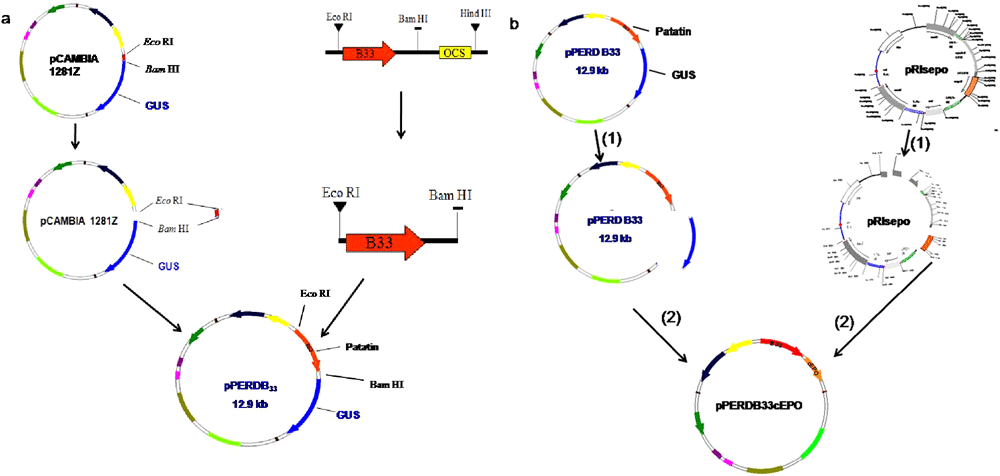
Figure 1. Schematic diagram of vector construction for tissue-specific expression of the EPO gene in potato plants.
a) Construction of vector containing patatin, pPERDB33 and, b) construction of vector containing EPO gene under patatin promoter.
Establishment of in vitro potato plant culture
Shoot cultures of the variety Kufri Bahar were established from sprouted buds excised from the tubers. The buds were surface sterilized with 0.1% mercuric chloride for 3 min followed by a rinse with 70% ethanol. After repeated washing with sterile distilled water, the buds were cultured on Murashige and Skoog (MS) medium supplemented with 0.1 µM GA3. The pH of the medium was adjusted to 5.7 and all the cultures were solidified with 0.8% agarose. The cultures were exposed to 16 hours light, which provided 1600 lux intensity of light, and maintained at 24 ± 2 °C temperature with 50–60% relative humidity. The buds developed into plantlets in 4 weeks. Nodes from these plantlets were cultured on MS medium containing 3% sucrose for raising the fresh cultures. Shoots from the fresh cultures were cultured into the MS medium supplemented with 8% sucrose and 6 µM BA to produce the microtuber. Inter-nodal segments, leaf discs and microtuber discs were routinely used for Agrobacterium-mediated transformation. To raise the shoots from inter-nodal segments, leaf discs and microtuber discs were used. Direct shoot regeneration was observed in all three explants in the two stage medium. In stage 1, all the explants were inoculated in PSRI 1 medium (MS medium + 1mg/l thiamine + 2% sucrose + 11µM zeatin + 1 µM NAA + 0.05 µM GA3 + 0.8% agar) for 20–30 days and then shifted to the PSRI 2 medium (MS medium + 1 mg/l thiamine + 2% sucrose + 9 μM zeatin + 0.1 μM NAA + 0.05 μM GA3). This medium was standardized for direct shoot regeneration from internodes by Millam (2006)26.
Transformation of potato plants
Inter-nodal segments, leaf discs and microtuber discs from in vitro grown plants were used for transformation and regeneration, essentially as described by Millam (2006)26 with some modifications. The procedure described by Millam (2006)26 for internodes was also used for leaf discs as well as microtuber discs. All three explants were incubated in liquid shoot regeneration medium (MS medium containing 1 mg/l thiamine + 2% sucrose + 11 µM zeatin +1 µM NAA + 0.05 µM GA3) for 2–3 hours in presence of 20 µM acetosyringone. After preconditioning, the explants were infected with Agrobacterium tumefaciens LBA4404 harboring the pPERDB33cEPO for 15 minutes. The explants were then blotted dry on sterile whatman No. 1 filter paper and were co-cultured on shoot regeneration medium containing 0.8% agar for 2 days. Following co-cultivation, the explants were transferred to shoot regeneration medium containing 0.8% agar and 500 mg/ml cefotaxime for 7 days for inhibition of Agrobacterium growth. After 7 days, explants were transferred to shoot regeneration medium containing 0.8% agar, 500 mg/ml cefotaxime and 7.5 mg/ml hygromycine for selection of transgenic shoots.
Analysis of transgenic plants
After selection of transformed plant in antibiotic selection medium, genomic DNA was isolated by using fresh leaf from the regenerated shoots27. Genomic DNA was used as a template DNA for the amplification of the cEPO gene. After transformation, T-DNA was integrated into the plant nuclear DNA. In order to confirm the presence of cDNA sequence of EPO in plant nuclear DNA, gene specific PCR was performed by using EPO cDNA-specific primer, forward primer: 5’ CCACCACGCCTCATCTGTGAC 3’ and reverse primer 5’ TCTGTCCCCTGTCCTGCAGGC 3’. PCR amplification was performed in 50 µl reaction containing primers (50 ng each), Taq DNA polymerase (1 unit), 200 µM dNTP, 1 × PCR buffer and 50 ng of genomic DNA as template amplifying a 408 bp fragment of the EPO gene. The PCR cycling conditions were set as initial melting at 95°C for 5 minutes, followed by 30 cycles of amplifications with each cycle consisting of the following steps: 95°C for 15 seconds, 68°C for 20 seconds and 72°C for 1 minute.
Results
To produce the EPO protein in a tuber-specific manner, patatin promoter was first excised and ligated into the backbone vector pCAMBIA1281Z to produce pPERDB33. The confirmation of pPERDB33 was done by RE digestion and agarose gel electrophoresis (figure 2).
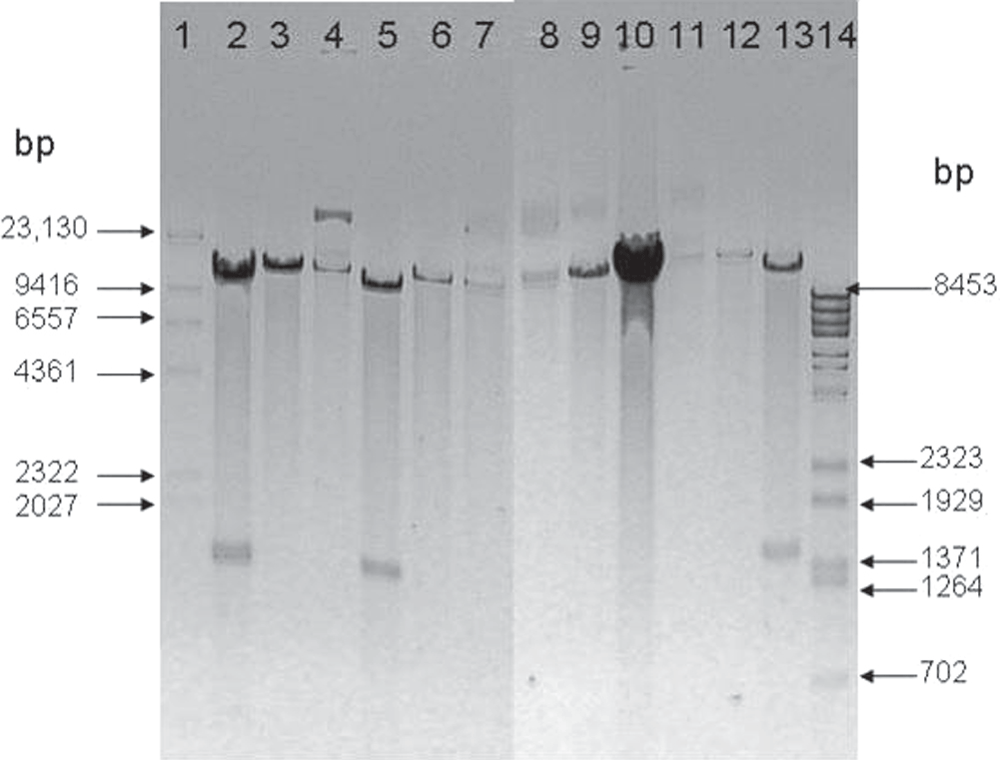
Figure 2. Confirmation of transformant by double RE digestion.
Lane 1 contains Marker (lamda DNA digested with Hind III), lane 2 contains Eco RI and Bam HI digested plasmid from clone 23, lane 3 contains Eco RI digested plasmid from clone 23, lane 4 contains undigested plasmid from clone 23, lane 5 contains Eco RI and Bam HI digested plasmid from clone 26, lane 6 contains Eco RI digested plasmid from clone 26, lane 7 contains undigested plasmid from clone 26, Lane 8 contains undigested pCAMBIA 1281Z, lane 9 contains Eco RI digested pCAMBIA 1281Z, lane 10 contains Eco RI and Bam HI digested pCAMBIA 1281Z, lane 11 contains undigested pBinB33, lane 12 contains Eco RI digested pBinB33, lane 13 contains Eco RI and Bam HI digested pBinB33 and lane14 contains Marker (lamda DNA digested with Bst EII).
After confirmation of the right clone having patatin prompter upstream to the GUS gene, the clone was maintained in E. coli. The Agrobacterium-mediated method was used for expression of the EPO gene in the potato plant. Plasmid pPERDB33, which contains the GUS gene downstream from the patatin promoter, was mobilized into Agrobacterium LBA4404 using tri-parental mating and confirmation of right clone, which was selected on the Luria agar plate containing rifampicin and chloramphenicol, and was done by the same method used for selection of the right clone in E. coli.
To clone the EPO protein into the vector pPERDB33 downstream to the patatin promoter the cDNA sequence of the human EPO gene was excised from plasmid pRISepo and ligated to the plasmid pPERDB33 in place of GUS. Confirmation of the right clone was done by RE digestion and agarose gel electrophoresis (Figure 3). The resultant plasmid which has the EPO gene downstream to the patatin promoter was named pPERDB33cEPO. pPERDB33cEPO was subsequently mobilized to the Agrobacterium LBA4404 using tri-parental mating and confirmed by RE digestion and agarose gel electrophoresis.
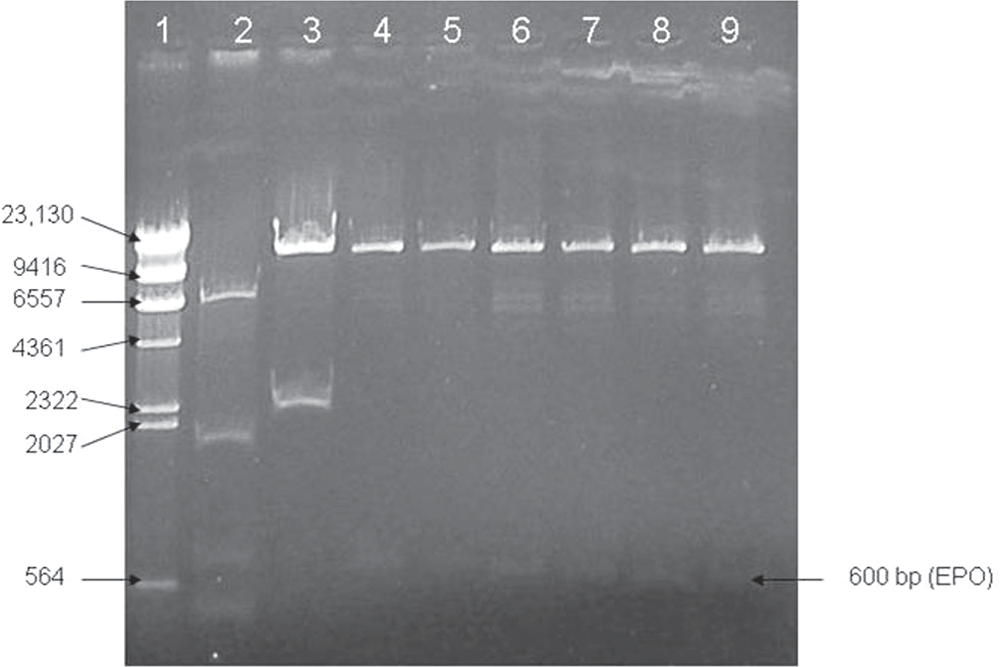
Figure 3. Confirmation of transformant (pPERDB33cEPO) by RE digestion.
Lane 1 contains marker (lamda DNA digested with Hind III marker of MBI Fermentas), lane 2 contains pRIsepo digested with Nco I and Bst EII, lane 3 contains pPERDB33 digested with Nco I and Bst EII and lanes 4 to 8 contain PCR positive clones.
The resultant plasmid pPERDB33cEPO was then introduced into potato plants by using Agrobacterium-mediated transformation. Transformed plants were selected on the PSRI medium containing 7.5 mg/L hygromycin and 500 mg/L cefotaxime. After 1 month, transgenic shoot formation was observed from the microtuber discs in the antibiotic selection medium (Figure 4). However, shoot formation was also observed from inter-nodal explants, but they did not survive for a longer time in the antibiotic stress medium.

Figure 4. Shoot formations from transformed microtuber discs.
Genomic DNA was isolated from the leaf of transformed plant. The relative quantity and quality of genomic DNA was analyzed using agarose gel electrophoresis. After transformation of the plant by the Agrobacterium method, the T-DNA segment containing the EPO gene was integrated into the plant nuclear genome. To investigate the stable integration of gene of interest in to plant nuclear genome, gene-specific PCR was done. Isolated genomic DNA from the transformed plant was used as template DNA. For gene-specific PCR analysis, EPO specifically primed along with the plasmid DNA containing the EPO sequence (pPERDB33cEPO) as a positive control was used.
After PCR amplification, the PCR product was analyzed using 1.5% agarose gel (Figure 5). A 100 bp DNA ladder was used as marker. A 408 bp band corresponding to EPO amplicon was observed in the gel (Figure 5, lane 4), which was similar to the amplicon obtained from pPERDB33cEPO used as a positive control (Figure 5, lane 5). From this result it was confirmed that the EPO sequence was successfully integrated into the plant nuclear genome.
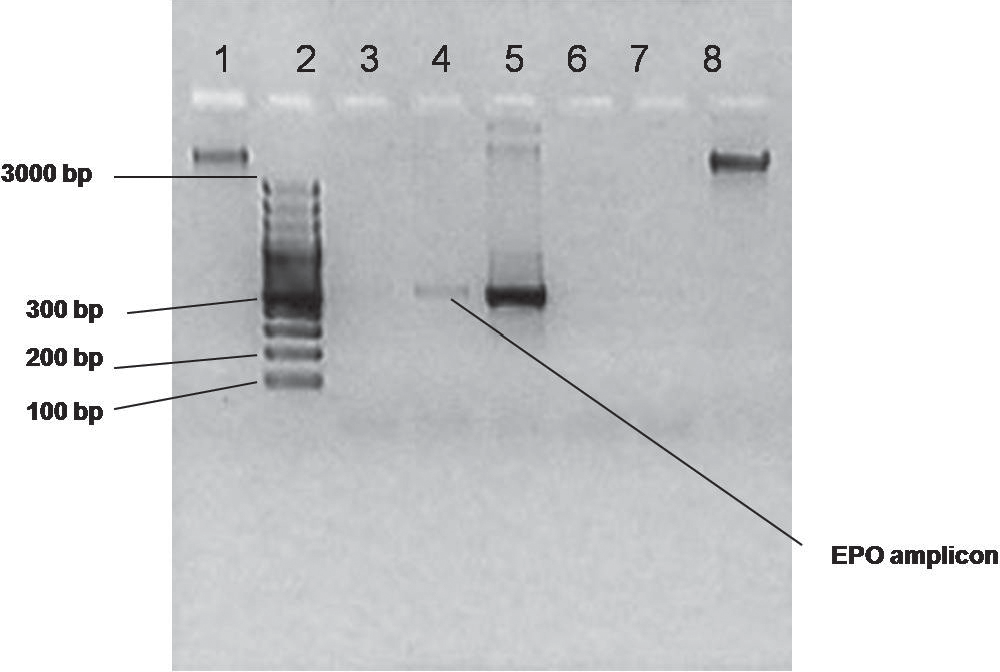
Figure 5. Analysis of PCR-positive transformed plants on 1.5% agarose gel.
Lane 1 contains Genomic DNA isolated from transformed plant 1, lane 2 contains DNA ladder as a marker (100 bp MBI fermentas), lane 3 and lane 4 contain PCR product of transformed plants, lane 5 contains positive control (PCR product of pPERDB33cEPO), lane 6 contains negative control (PCR product of pBSSK which does not contain EPO gene), lane 7 contains negative control of PCR reaction (master mix) and lane 8 contains genomic DNA isolate from transform plants.
Discussion
The aim of the present study was to develop a protocol for production of heterologous proteins in potato tuber. Vectors for the production of the erythropoietin gene under tissue-specific promoter and tissue culture techniques for regeneration of a whole transgenic potato plant from single explants were standardized successfully. Direct shoot regeneration from a number of explants like leaf discs, in vivo tuber discs, in vitro tuber discs and internodes were successfully achieved. A very low plant transformation efficiency and a high percentage of necrosis were observed using Agrobacterium-mediated plant transformation. To reduce the necrosis of explants and increase the transformation efficiency time of co-cultivation, inoculum of the bacteria as well as the time for pre-selection were optimized. Hygromycin which was used in selection media caused necrosis of explants even at lower concentrations. Transgenic potato plants were confirmed with the gene-specific PCR, which proved that our gene of interest successfully integrated into the desired plant nuclear genome. However the PCR-positive plant was not able to form rooting when transformed to the rooting medium containing hygromycin. This may be because the hygromycin concentration was lethal when it directly came into contact with the shoot.
Overall, the study demonstrates the feasibility of designing vectors to be used in creating transgenic potato plants for tissue-specific heterologous protein production. However, a lot remains to be done in optimizing the transformation and selection processes before the process can be used for large-scale production of heterologous proteins using tissue-specific expression in tuber of Solanum tuberosum.





Comments on this article Comments (0)