Background and aims
Despite continuous decline in incidence rates in both sexes, gastric cancer is still the fourth most common cancer worldwide, with 934,000 newly diagnosed cases per year1 and a vast annual death toll of more than 800,000, according to WHO 2004 statistics. With only little improvement over the past decades, the long-term survival from gastric cancer is poor, since patients are often diagnosed with advanced disease. In the USA, for example, five-year survival is 24%2. In a hope for an overall improvement in the forlorn statistics, worldwide efforts are invested to shorten the time-to-diagnosis. Stomach cancer screening has been first introduced in Japan in 1963, followed by The Republic of Korea in 1996, and has recently commenced in less developed countries, with Venezuela, Chile, and Costa Rica adapting the Japanese model to implement pilot screening routines. Notably, the fact that to date no randomized trial of stomach cancer screening has ever been conducted sets hurdles for reliable assessment of efficacy of such policy. Nevertheless, data from recent prospective studies shows reductions in mortality from gastric cancer among participants in screening programs in Japan and Costa Rica3–5. Importantly, in this regard, endoscopic procedures, although of undisputable great diagnostic value, face a lack of patient compliance, for being widely regarded as uncomfortable. Henceforth, to circumvent this obstacle, reliable biological markers, suitable for detection and monitoring of tumor growth in bodily fluids, have long been searched after, in both blood and gastric fluid. Understandably, being easily accessible, blood has long been the substance of choice for marker evaluation. However, as appears from the peer-reviewed sources, the vast majority of the proposed gastric cancer marker candidates share the lack of diagnostic potential. This flaw, expressed in low sensitivity and specificity parameters, obviously negates the idea of utilization of such markers in gastric cancer screening practice.
As new potential screening methodologies, such as capsule endoscopy6, arise, gastric fluid is becoming an attractive milieu for cancer diagnosis as it contains both secreted soluble and exfoliated cellular proteins from the entire gastric mucosa. Unfortunately, early reports on the diagnostic and prognostic utility of the preponderant indicators of neoplasia, such as carcinoembryonic antigen (CEA) and CA 19-9, yielded unsatisfactory results7–9, thus diverting researchers’ attention to other biomarkers, including CA72-4. Historically, following its identification [a sialosyl-2–6-α-N-acetylgalactosaminyl epitope of TAG-72 mucin;10–15], and characterization, the applicability of the marker to cancer detection was examined in several neoplastic conditions. As second generation high-affinity monoclonal antibody (recognizing a different epitope;16) has enabled establishment of a double-determinant immunoradiometric assay17,18 capable of detection of CA72-4 in bodily fluids of carcinoma patients, CA72-4 presence was assessed in the serum of patients diagnosed with gastrointestinal malignancies19–21. These studies disclosed a complementarity between CA72-4 and CEA, as the former was often elevated in samples from cancer patients in which the levels of the latter remained low. Moreover, serum CA72-4 in patients during post surgical follow-up was predictive of recurrent disease20. Several recently demonstrated lines of evidence also described CA72-4 as a potential serum and peritoneal wash-fluid marker of gastric malignant neoplasia, supreme to CEA (22; and references therein). In the present report we show that CA72-4, whose levels had to date been mainly assessed in serum samples23–26, has the potential to become a major biomarker in the gastric fluid-based gastric cancer diagnosis.
Methods
Settings
Collection of gastric juice samples and clinical data was performed at Israelitic Hospital (IH) in Hamburg, Germany, an academic hospital and tertiary referral center for patients with gastroenterological diseases. Immunological analysis was performed at the R&D laboratory of Novamed ltd., an ISO 9001-compliant facility, in Jerusalem, Israel.
Patients and experimental protocol
All patients older than 18 years of age undergoing an esophago-gastro-duodenoscopy (EGD) for clinical reasons were generally eligible for the study. Study participants gave written informed consent before any study related procedures were performed.
In all volunteers the EGD was performed as indicated clinically and according to routine procedures, including biopsies and interventional therapeutic measures, e.g. dilation therapy, if indicated. However, juice samples were only collected and analyzed if investigators could intubate the stomach and thoroughly aspirate gastric contents under visual control into separate vials before taking biopsies or performing other measures that might have altered gastric contents (e.g. rinsing of the mucosa with saline in order to improve visibility of potential mucosal alterations).
Volume and pH of each gastric juice sample were measured and recorded immediately, (pH was reassessed prior to immunological analysis). Subsequently, the pseudonymized samples were stored at -20°C until the end of the day and then transferred to -80°C for further storage until evaluation of biomarker concentration.
Collection of clinical data
Clinical data may be of pivotal importance for correct identification of biomarkers and was collected prospectively from all study participants. Data were pseudonymized and included the following information: results of endoscopic and histologic investigations, age, sex, height, weight, symptoms, time of last food and fluid intake, diet (e.g. vegetarian), concomitant medication (in particular intake of proton pump inhibitors (PPI)), alcohol consumption, smoking habits and relevant previous and concomitant diseases including abdominal surgery.
Definition of patient groups
Patient groups were defined according to the results of the endoscopic and histologic investigations:
A) Normal stomach: Normal EGD and normal histology according to representative biopsies from the antrum and the corpus.
B) Gastric inflammation: Endoscopic diagnosis of gastric ulcer(s), erosions or gastritis and/or histologic findings of more than mild gastritis (endoscopic diagnosis of gastritis based on reddening and swelling of the mucosa was only accepted if confirmed histologically).
C) Intestinal metaplasia: Histologic evidence of intestinal metaplasia, irrespective of other endoscopic and/or histologic findings (except gastric cancer).
D) Gastric cancer: Endoscopic and histologic evidence of gastric cancer, no previous therapy.
E) Miscellaneous: Other diseases of the stomach diagnosed endoscopically and/or by histology, or diseases of the esophagus or duodenum.
ELISA
Assays were performed by a laboratory technician blinded to patient clinical data, including diagnosis. Samples were analyzed, immediately following thawing, with CA72-4 ELISA assay (DRG instruments GmbH, Marburg, Germany), according to the manufacturer’s protocol, using 10 µl of sample. At least duplicate absorbance readings were obtained for each sample at 450nm wavelength using FL600 microplate reader with KC4™ data analysis software, (Bio-Tek® Instruments Inc., Winooski, VT, USA). Samples demonstrating high level of CA72-4 on the initial reading were assessed, in duplicates, up to 5 times on different days using a different assay plate and reagent set, depending on sample availability. Data collection, processing, and initial statistical analysis were performed on-site by a senior scientist, also blinded to patient clinical data. Data was then transferred to the IH site for detailed statistical analysis.
Statistical methods
Statistical analyses including ANOVA, Wilcoxon or Kruskal-Wallis test and univariate and multivariate linear regression analysis were performed using JMP software (version 6.0.3; SAS Institute Inc., Cary, NC, USA). Data are expressed as mean±SD or median with interquartile ranges depending on whether data were normally distributed or not. Multivariate linear regression analyses were used to investigate the influence of patient grouping and clinical parameters on CA72-4 concentrations in gastric juice. For the multivariate analyses, manual stepwise model building was performed and the following parameters were tested as predictors: age, gender, BMI, Helicobacter pylori (H. pylori) status (according to histology), smoking habits (never vs. active or ex-smoker), alcohol intake (never vs. current or ex-alcohol intake), PPI dose (in multiples of standard dose), endoscopic evidence of gastric bleeding and histologic diagnosis of gastric carcinoma, intestinal metaplasia, gastric inflammation and nonmalignant histologic alterations of the gastric mucosa.
Results
Study participants
Overall 380 patients consented to participate in the study. Collection of gastric juice and clinical data was performed in 262 patients, in most of the others no juice samples could be obtained because the stomach contained insufficient amounts of fluid to be aspirated endoscopically or solids that could not be aspirated. In a minority of patients clinical data were insufficient for study purposes.
CA72-4 concentrations were measured in 176 gastric juice samples, based on sample volume sufficiency, from subjects with normal stomach (N=28), gastric inflammation (N=58), intestinal metaplasia (N=26), gastric carcinoma and no previous therapy (N=8) and patients with miscellaneous diseases (N=56). 108 out of 176 patients were female. Mean age of the patients was 60.8±16.8 years, mean BMI was 25.1±4.9 kg/m2.
CA72-4 concentrations in gastric juice
CA72-4 concentrations in gastric juice ranged from 0.3 to 287 U/ml (Figure 1). Median CA72-4 concentration was 35.5 [19–68.5] U/ml. There was a marked and highly significant difference between the CA72-4 concentrations observed for the various diagnostic groups (p=0.0005). While CA72-4 concentrations in gastric juice were similar in patients with an endoscopically and histologically normal stomach, gastric inflammation, intestinal metaplasia of the gastric mucosa or miscellaneous diseases, patients with gastric cancer had markedly elevated CA72-4 levels (Figure 2). Compared with all other participants, median CA72-4 concentrations in gastric juice of cancer patients were increased about fourfold (144.5 [54.0–220.3] U/ml vs. 34.5 [18.1–64.0] U/ml, p=0.001) (Figure 2). Six out of eight patients with gastric cancer and 18 out of 168 patients with other diagnoses had CA72-4 concentrations above 100 U/ml. Thus, at this cut-off level elevated CA72-4 concentrations had 75% sensitivity and 89% specificity for detection of gastric cancer.
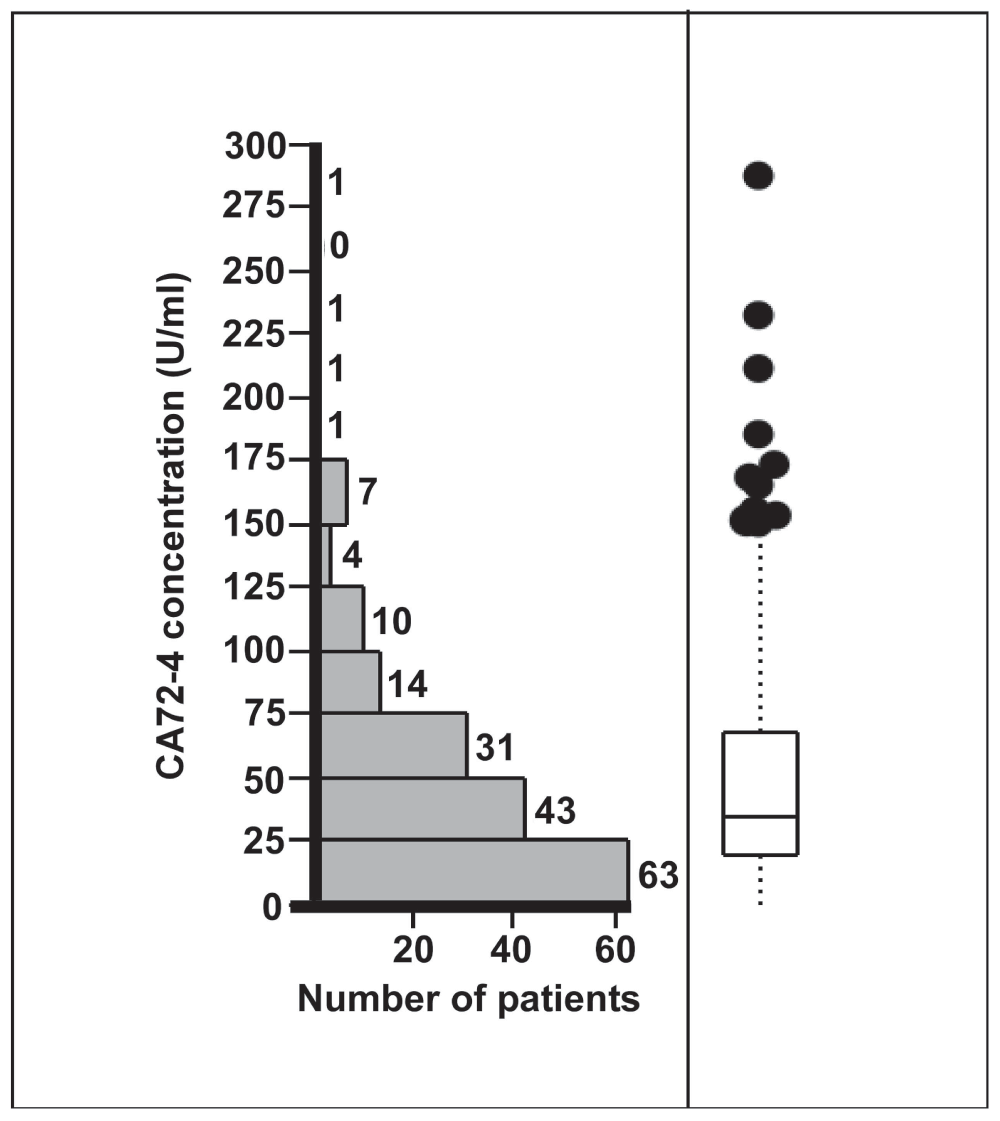
Figure 1. Distribution of CA72-4 concentrations in the gastric juice of 176 patients undergoing routine esophago-gastro-duodenoscopy.
Individual values ranged from 0.3 to 278 U/ml. The box plot shows median CA72-4 concentration (35.5 U/ml) and interquartile ranges (19–68.5 U/ml).
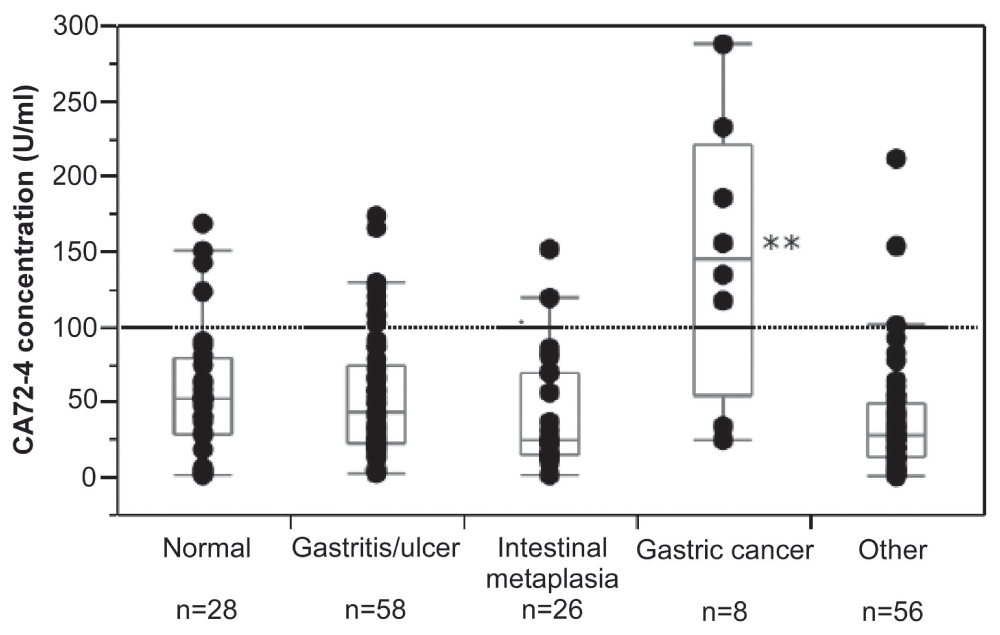
Figure 2. Concentration of CA72-4 in gastric juice of various groups of patients undergoing routine esophago-gastro-duodenoscopy (EGD; 176 in total).
Patients with an endoscopically and histologically normal stomach, gastric inflammation, intestinal metaplasia of the gastric mucosa or miscellaneous diseases had similar CA72-4 concentrations. By contrast, in patients with gastric cancer CA72-4 concentrations were significantly elevated compared with all other groups (**p<0.001). The figure shows individual values and box-plot diagrams for the various groups. At a cut-off level of 100 U/ml (dotted line), elevated CA72-4 concentrations had 75% sensitivity and 89% specificity for detection of gastric cancer.
Univariate linear regression analysis
In the univariate linear regression analysis, the diagnosis of gastric cancer significantly predicted increased CA72-4 concentrations in gastric juice (p<0.0001). Age or smoking habits were predictors of borderline significance for increased CA72-4 levels in older subjects and smokers (p=0.051 and p=0.090, respectively).
CA-72-4 levels were not significantly predicted by diagnosis of intestinal metaplasia (p=0.964), gastric inflammation (p=0.656), H. pylori status (p=0.874), gastric bleeding (p=0.491), sex (p=0.206), BMI (p=0.218), alcohol intake (p=0.857), PPI dose (p=0.252) or pH of the aspirate (p=0.426). If patients with gastric inflammation, intestinal metaplasia and miscellaneous diseases of the gastrointestinal tract were combined to one group of patients with pathologies other than gastric carcinoma, prevalence of this diagnosis did also not predict CA-72-4 concentrations in gastric fluid in the univariate analysis (p=0.226).
Multivariate linear regression analysis
Multivariate linear regression analysis confirmed that CA72-4 concentration in gastric juice was significantly predicted by diagnosis of gastric carcinoma adjusted for age, smoking habits, H. pylori status, PPI dose, and pH of the aspirate (R2=0.27, p<0.0001). In this model, diagnosis of gastric carcinoma had by far the greatest influence (p<0.0001). Age (p=0.033) and smoking status (p=0.002) were additional independent significant predictors of CA72-4 concentrations. The other parameters achieved only borderline significance (p=0.079 to p=0.138), but the overall significance of the model was reduced when these parameters were not taken into account.
Multivariate linear regression analysis further revealed that CA72-4 concentrations were significantly predicted by pathologies other than gastric carcinoma adjusted for H. pylori and smoking status, age, PPI dose, and pH of the aspirate. However, gastrointestinal diseases other than gastric carcinoma had opposite (decreased levels in patients with such diseases) and much weaker effects (R2=0.07, p=0.028) on CA72-4 concentrations compared with diagnosis of gastric cancer. In this model, diagnosis of H. pylori and smoking status were independent significant predictors of CA72-4 concentrations (p=0.0384 and p=0.009, respectively), while the other parameters achieved borderline significance (p≥0.073).
Discussion
The combination of a relatively high incidence, especially in northeast Asia, and a frequently late stage diagnosis of gastric cancer, due to the patient- or physician-side misinterpretation of symptoms, has proven deadly over the years, making the disease the second most frequent cause of cancer death worldwide27,28. Generally, the cancer progression is aggressive upon late stage diagnosis with 5-year survival rates usually less than 30%. The existing means of screening are very accurate in providing a diagnosis, but face low patient-side compliance due to known discomfort associated with endoscopic procedures, thus imposing indirect constraints on the diagnostic value of fibreoptic endoscopy. The emerging capsule endoscopy, although holding a promise to circumvent the issue of compliance, still lacks diagnostic capabilities beyond visual identification of lesions. Thus, it will inevitably, require a reliable substitute for histological analysis, readily available through biopsy collection using a “conventional” endoscope, to outcompete the latter in screening efficiency, simplicity, and time-to-diagnosis. One such alternative is “lab-on-capsule” cancer marker-based molecular recognition of gastric malignancies. This option can be made possible through utilization of one or more marker(s) featuring high sensitivity and specificity in detection of gastric malignant tumors. In this work, we embarked upon identification of such a marker through assessment of the above mentioned parameters for a selected group of cancer markers, levels of which were measured in the gastric juice collected from patients with various disorders of the upper GIT, as well as in the gastric juice of patients in whom no disease was detected by either or both EGD and histopathology. During this study we have established several ELISA assays for markers previously implicated in relation with gastric cancer, such as: CEA7–9,23, pepsinogen II (PGII;29–33), regenerating islet-derived family, member 4 (RegIV;34,35), and cytokeratin 8 (CK8;36–38), and have also made use of commercially available assays for CA 19-97–9,23, Gastrin1730, and pepsinogen I (PGI;31–33). However, in our preliminary large-scale sampling analysis we failed to find any difference between the levels of these markers (including PGI/PGII ratio) in the gastric juice of cancer and non-cancer patients (data not shown). Quite the reverse, data generated in the course of this study points to a great diagnostic potential of CA72-4 direct measurements in gastric fluid. Notably in this regard, the CA72-4 concentrations in the gastric fluid of the majority of cancer patients were prominently elevated, compared to those measured in the gastric fluid obtained from patients with a completely normal stomach, patients with gastric inflammation, intestinal metaplasia or miscellaneous diseases of the upper gastrointestinal tract, including other malignant tumors.
One of the most obdurate hurdles to detection of cancer markers in gastric juice is the hostility of the gastric milieu imposed, among other factors, by high proteolytic activity and high acidity. Univariate linear regression analysis did not reveal a significant association between pH of gastric juice and CA72-4 concentrations. In multivariate linear regression analysis the “protective” effect of higher pH on CA72-4 concentrations was small, and failed to show statistical significance. In this regard, the apparent superiority of CA72-4 over other potential biomarkers in gastric juice may be speculated to stem from its relative stability in a wide range of pH, possibly due to the nature of the detected epitopes.
At a cut-off level of 100 U/ml CA72-4 had a sensitivity of 75% and a specificity of 89% for detection of gastric cancer. These data are also encouraging, although, with respect to the sensitivity parameter, the rather low number of cancer patients included in the study limits reliability of these findings and, generally, represents the most important drawback of our study. Gastric cancer was newly diagnosed in 8 out of 176 patients (5%), a proportion somewhat higher than that of cancer diagnoses expected in unselected patients undergoing EGD because of dyspeptic symptoms [1–2%;39). Notably, the IH is a tertiary referral center for patients with gastrointestinal diseases where gastric cancer patients are observed much more frequently. However, in most patients satisfactory endoscopic and histologic investigations have been performed prior to referral to IH. The experimental design did not allow performance of an EGD for reasons other than clinical, hence, certain patients could not be included in the study. Moreover, for final analysis of data only patients with newly diagnosed disease were selected in order to limit potential confounders. Interestingly, an ostensible decrease in CA72-4, measured in a single patient concomitantly with cancer detection (150 U/ml), was observed following chemotherapy (87 U/ml). Thus, in the future, effort should be invested in further exploration of this phenomenon and the possibility that CA72-4 may also be used to monitor treatment efficiency.
Contrary to sensitivity, the specificity data are based on analysis of a large number of gastric juice samples from patients with completely normal gastric findings or various gastric or other diseases, advocating for data reliability. We observed a trend towards lower intragastric CA72-4 concentrations in patients with pathologies other than gastric cancer and an accordingly high specificity of nearly 90% for CA72-4-based detection of gastric cancer at the cut-off level chosen. This is particularly encouraging for a putative screening marker, as low specificity would be associated with a large number of futile invasive diagnostic tests in patients that are, in fact, free from gastric cancer.
In conclusion, work will be needed to accurately establish the precise sensitivity and the cut-off level for cancer detection. However, more than a fourfold, and thus a highly biologically significant, increase in the mean value of CA72-4 in the cancer group patients in this study, compared to all other participants, along with the apparent high specificity of the marker-based cancer detection, underscore the validity of our findings, and suggest that CA72-4 may contribute to real-time detection of gastric cancer in the future.
Consent
Study participants gave written informed consent before any study related procedures were performed.
Author contributions
Jutta Keller – acquisition of data, study concept and design, analysis and interpretation of data, drafting of the manuscript, statistical analysis.
Ella Reiss-Sklan – acquisition of data, analysis and interpretation of data, statistical analysis.
Miri Refael – acquisition of data.
Viola Andresen – statistical analysis.
Yael Herman-Levy – acquisition of data.
Igor Ruvinsky – acquisition of data, study concept and design, analysis and interpretation of data, drafting of the manuscript, statistical analysis, study supervision.
Jutta Keller and Ella Sklan have contributed equally to this work.
Competing interests
No competing interests were disclosed.
Grant information
This work was supported in part by CORDIS FP6 grant (#37362) for project LSHB-CT-2006-037362.
Faculty Opinions recommendedReferences
- 1.
Parkin DM, Bray F, Ferlay J, et al.:
Global cancer statistics, 2002.
CA Cancer J Clin.
2005; 55(2): 74–108. PubMed Abstract
| Publisher Full Text
- 2.
Ries LAG MD, Krapcho M, Mariotto A, et al.:
SEER Cancer Statistics Review 1975–2004. 2007. Reference Source
- 3.
Lee KJ, Inoue M, Otani T, et al.:
Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan.
Int J Cancer.
2006; 118(9): 2315–21. PubMed Abstract
| Publisher Full Text
- 4.
Mizoue T, Yoshimura T, Tokui N, et al.:
Prospective study of screening for stomach cancer in Japan.
Int J Cancer.
2003; 106(1): 103–7. PubMed Abstract
| Publisher Full Text
- 5.
Rosero-Bixby L, Sierra R:
X-ray screening seems to reduce gastric cancer mortality by half in a community-controlled trial in Costa Rica.
Br J Cancer.
2007; 97(7): 837–43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 6.
Moglia A, Menciassi A, Dario P, et al.:
Capsule endoscopy: progress update and challenges ahead.
Nat Rev Gastroenterol Hepatol.
2009; 6(6): 353–62. PubMed Abstract
| Publisher Full Text
- 7.
Muretto P, Graziano F, Staccioli MP, et al.:
An endogastric capsule for measuring tumor markers in gastric juice: an evaluation of the safety and efficacy of a new diagnostic tool.
Ann Oncol.
2003; 14(1): 105–9. PubMed Abstract
| Publisher Full Text
- 8.
Duraker N, Naci Celik A, Gencler N, et al.:
The prognostic significance of gastric juice CA 19-9 and CEA levels in gastric carcinoma patients.
Eur J Surg Oncol.
2002; 28(8): 844–9. PubMed Abstract
| Publisher Full Text
- 9.
Tocchi A, Costa G, Lepre L, et al.:
The role of serum and gastric juice levels of carcinoembryonic antigen, CA19.9 and CA72.4 in patients with gastric cancer.
J Cancer Res Clin Oncol.
1998; 124(8): 450–5. PubMed Abstract
| Publisher Full Text
- 10.
Colcher D, Hand PH, Nuti M, et al.:
A spectrum of monoclonal antibodies reactive with human mammary tumor cells.
Proc Natl Acad Sci U S A.
1981; 78(5): 3199–203. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Johnson VG, Schlom J, Paterson AJ, et al.:
Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3.
Cancer Res.
1986; 46(2): 850–7. PubMed Abstract
- 12.
Kjeldsen T, Clausen H, Hirohashi S, et al.:
Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2----6-alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope.
Cancer Res.
1988; 48(8): 2214–20. PubMed Abstract
- 13.
Thor A, Ohuchi N, Szpak CA, et al.:
Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3.
Cancer Res.
1986; 46(6): 3118–24. PubMed Abstract
- 14.
Thor A, Viglione MJ, Muraro R, et al.:
Monoclonal antibody B72.3 reactivity with human endometrium: a study of normal and malignant tissues.
Int J Gynecol Pathol.
1987; 6(3): 235–47. PubMed Abstract
| Publisher Full Text
- 15.
Wolf BC, D’Emilia JC, Salem RR, et al.:
Detection of the tumor-associated glycoprotein antigen (TAG-72) in premalignant lesions of the colon.
J Natl Cancer Inst.
1989; 81(24): 1913–7. PubMed Abstract
| Publisher Full Text
- 16.
Muraro R, Kuroki M, Wunderlich D, et al.:
Generation and characterization of B72.3 second generation monoclonal antibodies reactive with the tumor-associated glycoprotein 72 antigen.
Cancer Res.
1988; 48(16): 4588–96. PubMed Abstract
- 17.
Gero EJ, Colcher D, Ferroni P, et al.:
CA 72-4 radioimmunoassay for the detection of the TAG-72 carcinoma-associated antigen in serum of patients.
J Clin Lab Anal.
1989; 3(6): 360–9. PubMed Abstract
| Publisher Full Text
- 18.
Ferroni P, Szpak C, Greiner JW, et al.:
CA 72-4 radioimmunoassay in the diagnosis of malignant effusions. Comparison of various tumor markers.
Int J Cancer.
1990; 46(3): 445–51. PubMed Abstract
| Publisher Full Text
- 19.
Byrne DJ, Browning MC, Cuschieri A, et al.:
CA72-4: a new tumour marker for gastric cancer.
Br J Surg.
1990; 77(9): 1010–3. PubMed Abstract
| Publisher Full Text
- 20.
Guadagni F, Roselli M, Amato T, et al.:
Tumor-associated glycoprotein-72 serum levels complement carcinoembryonic antigen levels in monitoring patients with gastrointestinal carcinoma. A longitudinal study.
Cancer.
1991; 68(11): 2443–50. PubMed Abstract
| Publisher Full Text
- 21.
Ohuchi N, Takahashi K, Matoba N, et al.:
Comparison of serum assays for TAG-72, CA19-9 and CEA in gastrointestinal carcinoma patients.
Jpn J Clin Oncol.
1989; 19(3): 242–8. PubMed Abstract
- 22.
Fernandes LL, Martins LC, Nagashima CA, et al.:
CA72-4 antigen levels in serum and peritoneal washing in gastric cancer. Correlation with morphological aspects of neoplasia.
Arq Gastroenterol.
2007; 44(3): 235–9. PubMed Abstract
| Publisher Full Text
- 23.
Wobbes T, Thomas CM, Segers MF, et al.:
Evaluation of seven tumor markers (CA 50, CA 19-9, CA 19-9 TruQuant, CA 72-4, CA 195, carcinoembryonic antigen, and tissue polypeptide antigen) in the pretreatment sera of patients with gastric carcinoma.
Cancer.
1992; 69(8): 2036–41. PubMed Abstract
- 24.
Klug TL, Sattler MA, Colcher D, et al.:
Monoclonal antibody immunoradiometric assay for an antigenic determinant (CA 72) on a novel pancarcinoma antigen (TAG-72).
Int J Cancer.
1986; 38(5): 661–9. PubMed Abstract
| Publisher Full Text
- 25.
Fernandez-Llamazares J, Pinol M, Encabo G, et al.:
[Tumor markers and gastric cancer. TAG-72 is the most useful].
Med Clin (Barc).
1998; 111(20): 800. PubMed Abstract
- 26.
Joypaul B, Browning M, Newman E, et al.:
Comparison of serum CA72-4 and CA 19-9 levels in gastric cancer patients and correlation with recurrence.
Am J Surg.
1995; 169(6): 595–9. PubMed Abstract
| Publisher Full Text
- 27.
New hope for advanced gastric cancer.
Lancet Oncol.
2010; 11(3): 211. PubMed Abstract
| Publisher Full Text
- 28.
Cappellani A, Zanghi A, Di Vita M, et al.:
Clinical and biological markers in gastric cancer: update and perspectives.
Front Biosci (Schol Ed).
2010; 2: 403–12. PubMed Abstract
| Publisher Full Text
- 29.
Ohata H, Oka M, Yanaoka K, et al.:
Gastric cancer screening of a high-risk population in Japan using serum pepsinogen and barium digital radiography.
Cancer Sci.
2005; 96(10): 713–20. PubMed Abstract
| Publisher Full Text
- 30.
Cao Q, Ran ZH, Xiao SD, et al.:
Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies.
J Dig Dis.
2007; 8(1): 15–22. PubMed Abstract
| Publisher Full Text
- 31.
Yoshihara M, Sumii K, Haruma K, et al.:
Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects.
Am J Gastroenterol.
1998; 93(7): 1090–6. PubMed Abstract
| Publisher Full Text
- 32.
Oishi Y, Kiyohara Y, Kubo M, et al.:
The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study.
Am J Epidemiol.
2006; 163(7): 629–37. PubMed Abstract
| Publisher Full Text
- 33.
Konishi N, Matsumoto K, Hiasa Y, et al.:
Tissue and serum pepsinogen I and II in gastric cancer identified using immunohistochemistry and rapid ELISA.
J Clin Pathol.
1995; 48(4): 364–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 34.
Takehara A, Eguchi H, Ohigashi H, et al.:
Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4.
Cancer Sci.
2006; 97(11): 1191–7. PubMed Abstract
| Publisher Full Text
- 35.
Mitani Y, Oue N, Matsumura S, et al.:
Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy.
Oncogene.
2007; 26(30): 4383–93. PubMed Abstract
| Publisher Full Text
- 36.
Takikawa M, Akiyama Y, Maruyama K, et al.:
Proteomic analysis of a highly metastatic gastric cancer cell line using two-dimensional differential gel electrophoresis.
Oncol Rep.
2006; 16(4): 705–11. PubMed Abstract
- 37.
Cheng Y, Zhang J, Li Y, et al.:
Proteome analysis of human gastric cardia adenocarcinoma by laser capture microdissection.
BMC Cancer.
2007; 7: 191. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 38.
He QY, Cheung YH, Leung SY, et al.:
Diverse proteomic alterations in gastric adenocarcinoma.
Proteomics.
2004; 4(10): 3276–87. PubMed Abstract
| Publisher Full Text
- 39.
Malfertheiner P, Holtmann G, Peitz U, et al.:
[Guidelines of the German Society of Digestive and Metabolic Diseases for treatment of dyspepsia].
Z Gastroenterol.
2001; 39(11): 937–56 [Article in German]. PubMed Abstract
| Publisher Full Text


Comments on this article Comments (0)