Case report
In November 2003, a 39 year old male patient was diagnosed with a locally advanced gastric adenocarcinoma. The diagnosis and tumor stage were confirmed by gastrocopy, endoscopic ultrasound (EUS = u-staging), computer tomography (CT) scans of the thorax/abdomen and a bone scintigraphy. Gastroscopic biopsies revealed a Helicobacter pylori-negative gastric adenocarcinoma, diffuse type G3. According to the TNM/UICC staging system the carcinoma stage was cT4N3M0 or stage IV, respectively1. We found an invasion of the colon transversum and more than sixteen significantly enlarged lymph nodes (Figure 1a). We did not test the Her-2-neu status in 2003. Multdisciplinary treatment planning included preoperative chemotherapy followed by operation and postoperative chemotherapy in curative intention. The patient suffered from a severe hypertensive cardiomyopathy, thus he was not eligible for an anthracycline containing chemotherapy.
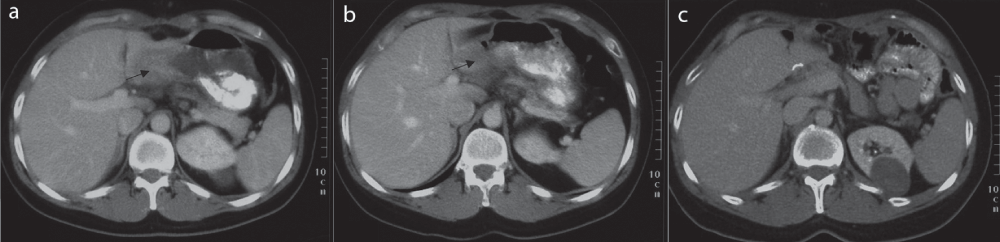
Figure 1. Abdominal CT scans.
a) before treatment December 2003 b) after partial remission May 2004 c) after complete remission November 2005.
After six weeks on PLF (cisplatin 50 mg/m2 every two weeks, weekly folinic acid 500 mg/m2 and 5-FU 2000 mg/m2/24h), we found no response. We changed to the FLI-regiment (weekly folinic acid 500 mg/m2, 5-FU 2000 mg/m2/24h, irinotecan 80 mg/m2), there was no response after six weeks as well. After applying two cycles of DC (docetaxel 50 mg/m2 d 1 + 15, cisplatin 50 mg/m2 d 1 + 15, every four weeks), we detected a partial remission on endoscopy and abdominal CT (Figure 1b). In June 2004, a gastrectomy with a D2 lymph node dissection was performed resulting in a R0 status and a pathologically confirmed complete remission (CR). The patient was in good postoperative condition, he tolerated two further postoperative cycles of DC well without more than grade-2-toxicity. In September 2004, the patient underwent a laparotomy due to an acute bowel obstruction caused by adhesive bands after previous abdominal surgery. No carcinoma was detected macroscopically or histologically (Figure 1c). Up to this day, more than thirteen years after the diagnosis, the tumor is still in CR. The patient’s quality of life is not compromised in any way (ECOG performance status 0).
Using the TNM/UICC-classification of gastric cancer 2010, our patient would be classified as stage cT4bN3bM0, stage IIIc2.
Discussion
The 5-year median overall survival rate for stage III and IV patients receiving perioperative chemotherapy is 39.1% and 5.2% after surgical resection alone3–5. Since 2010, the standard of care in locally advanced gastric cancer (uT3-T4) is three praeoperative cycles of an anthracycin containing polychemotherapy like epirubicine, cisplatin and 5-FU (ECF) followed by three postoperative cycles. This kind of treatment improved 5-year survival from 23% with surgery alone to 36.3%6,7. There are no sufficient data from randomised phase III studies to strongly recommend perioperative chemotherapy for patients with uT2 carcinomas8.
Two preoperative cycles of PLF seem to be as effective as preoperative ECF9,10. In advanced gastric cancer, polychemotherapy offers a survival advantage over single-agent therapy. Comparing 5-FU/cisplatin-containing regiments with versus without anthracyclins and 5-FU/anthracyclin-containing combinations with or without cisplatin there is a significant survival benefit for 5-FU + anthracyclins + cisplatin. In this analysis, secondary resectability in locally advanced disease was not reported11.
In metastatic gastric adenocarcimomas and adenocarcinomas of the gastrooesophaeal junction 5-FU can be substituted by capecitabine without compromising the results, so capecitabine may be used instead of 5-FU in the perioperative setting in combination with cisplatin (XP) or with epirubicine and cisplatin (ECX)12–14. In metastatic disease oxaliplatin has been tested as a substitute for cisplatin in combination chemotherapy with similar results13,15. The use of oxaliplatin can be recommended for patients suffering from renal insufficiency or patients with severe adverse events after cisplatin treatment8. Irinotecan or Docetaxel combinations have not been established for the perioperative treatment of gastric cancer in 2004. Both drugs were used in combinations for palliative therapy only16,17.
At the ASCO meeting in 2011 the FLOT3 trial15 has been presented:
Patients with untreated operable gastric adenocarcinoma received four preoperative cycles of FLOT (5-FU, leucovorin, oxaliplatin, docetaxel) and underwent surgical resection (D2 resection) followed by four postoperative cycles. Patients with limited metastatic disease (distant intra-abdominal lymph nodes and/or a maximum of one organ involved in metastatic disease, ECOG-PS < 1) received eight cycles and underwent surgical resection when a complete macroscopic resection seemed possible. Surgical treatment was conducted in 95.7% and 42.64% of the patients, respectively. For operable patients the median overall survival was not reached, for patients with limited disease the median overall survival was 18.6 months; there were also significant differences in progression-free survival (p < 0.001). Grade 3–4 toxicities were similar among the groups and occurred in 70.6% vs. 72.1% of the patients, respectively15.
In gastric adenocarcinoma or adenocarcinoma of the gastrooesophageal junction stage uT2 a perioperative chemotherapy can be considered (Level of evidence 1b), in stage uT3 and in resectable uT4a adenocarcinomas patients should receive a pre- and postoperative polychemotherapy (Level of evidence 1b)8.
Our patient may be one of the rare cases with advanced gastric cancer cured by perioperative third line chemotherapy and surgery.
Consent
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
Author contributions
IM found the case in his clinic. AD, ER, IM and HFK collected data. IM drafted the manuscript. DM rewiewed the literature and revised the manuscript critically. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Competing interests
No competing interests were disclosed.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Faculty Opinions recommendedReferences
- 1.
Sobin LH, Wittekind C:
TNM-classification of malignant tumors. 6th ed. New York: Springer2002. Reference Source
- 2.
Sobin LH, Gospodarowicz MK, Wittekind C, et al.:
TNM-classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell2010. Reference Source
- 3.
Wang W, Li YF, Sun XW, et al.:
Prognosis of 980 patients with gastric cancer after surgical resection.
Chin J Cancer.
2010; 29(11): 923–930. PubMed Abstract
| Publisher Full Text
- 4.
Boige V, Pignon JP, Saint-Aubert JB, et al.:
Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus.
J Clin Oncol.
2007; 25(Suppl 18S): abstr 4510. Reference Source
- 5.
Kelsen DP, Ginsberg R, Pajak TF, et al.:
Chemotherapy followed by surgery compared with surgery alone for localized esophaegal cancer.
N Engl J Med.
1998; 339(27): 1979–1984. PubMed Abstract
| Publisher Full Text
- 6.
Okines A, Verheij M, Allum W, et al.:
Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up.
Ann Oncol.
2010; 21(Suppl 5): v50–54. PubMed Abstract
| Publisher Full Text
- 7.
Cunningham D, Allum WH, Stenning SP, et al.:
Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer.
N Engl J Med.
2006; 355(1): 11–20. PubMed Abstract
| Publisher Full Text
- 8.
Moehler M, Al-Batran SE, Andus T, et al.:
German S3-guideline "Diagnosis and treatment of esophagogastric cancer".
Z Gastroenterol.
2011; 49(4): 461–531. PubMed Abstract
| Publisher Full Text
- 9.
Schuhmacher C, Schlag P, Lordick F, et al.:
Neoadjuvant chemotherapy versus surgery alone for locally advanced adenocarcinoma of the stomach and cardia: Randomized EORTC phase III trial 40954.
J Clin Oncol.
2009; 27(Suppl 15s): abstr 4510. Reference Source
- 10.
Schuhmacher C, Gretschel S, Lordick F, et al.:
Neoadjuvant chemotheraphy compared with surgery alone for locally advanced cancer of the stomach and cardia: European organisation for research and treatment of cancer randomized trial 40954.
J Clin Oncol.
2010; 28(35): 5210–5218. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Wagner AD, Unverzagt S, Grothe W, et al.:
Chemotherapy for advanced gastric cancer.
Cochrane Database Syst Rev.
2010; (3): CD004064. PubMed Abstract
| Publisher Full Text
- 12.
Kang YK, Kang WK, Shin DB, et al.:
Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial.
Ann Oncol.
2009; 20(4): 666–673. PubMed Abstract
| Publisher Full Text
- 13.
Cunningham D, Starling N, Rao S, et al.:
Capecitabine and oxaliplatin for advanced esophagogastric cancer.
N Engl J Med.
2008; 358(1): 36–46. PubMed Abstract
| Publisher Full Text
- 14.
Okines AF, Norman AR, McCloud P, et al.:
Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer.
Ann Oncol.
2009; 20(9): 1529–1534. PubMed Abstract
| Publisher Full Text
- 15.
Al-Batran SE, Hartmann JT, Probst S, et al.:
Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie.
J Clin Oncol.
2008; 26(9): 1435–1442. PubMed Abstract
| Publisher Full Text
- 16.
Pozzo C, Bugat R, Peschel C, et al.:
Irinotecan in combination with CDDP or 5-FU and folinic acid is active in patients with advanced gastric or gastro-oesophageal junction adenocarcinoma: Final results of a randomised phase II study.
Proc Am Soc Clin Oncol.
2001; 20: abstr 531.
- 17.
Roth AD, Maibach R, Falk S, et al.:
Docetaxel-cisplatin-5FU (TCF) versus docetaxel-cisplatin (TC) versus epirubicin-cisplatin-5FU (ECF) as systemic treatment for advanced gastric carcinoma (AGC): A randomized phase II trial of the Swiss Group for Clinical Cancer Research (SAKK).
J Clin Oncol.
2004; 22(Suppl (14s) (July 15 Supplement)): abstr 4020. Reference Source

Comments on this article Comments (0)