Introduction
Acute Myelogenous Leukemia (AML) is a group of hematogenous neoplasms characterized by clonal proliferation of myeloid precursors with a reduced capacity to differentiate into more mature cellular elements1. As a result, there is an accumulation of leukemic blasts or immature forms in the bone marrow, peripheral blood, and occasionally in other tissues, with a variable reduction in the production of normal red blood cells, platelets, and mature granulocytes. The increased production of malignant cells, along with a reduction in these mature elements, result in a variety of systemic consequences including anemia, bleeding, and an increased risk of infection1. Less than 1 percent of patients present with prominent extramedullary disease2. These extramedullary manifestations can manifest simultaneously with, or precede, bone marrow involvement. Sites of isolated expression include bone, periosteum, soft tissues, and lymph nodes, and less commonly the orbit, intestine, mediastinum, epidural region, uterus, and ovary2. To our knowledge this is one of the few reported cases of pleural effusion as the initial manifestation of AML.
Case report
A 66 year old man with a long-standing history of mild to moderate asthma and arterial hypertension was evaluated for a worsening productive cough of clear sputum, dyspnea, wheezing, and unintentional weight loss of approximately thirty pounds. The patient denied fever, chills, hemoptysis, night sweats, chest pain, or exposure to sick contacts. His medications were frequent use of short acting β-agonist with minimal resolution of symptoms.
On physical examination, the patient was alert but in mild respiratory distress, afebrile without hemodynamic compromise. The cardiac examination was normal; pulmonary examination revealed diffusely decreased breathing sounds, inspiratory crackles, and dullness to percussion, decreased fremitus and egophony in up to two thirds of the left lung field. There was no use of accessory muscles and oxygen saturation was 90% with the patient breathing ambient air. Neither lymphadenopathy nor organomegaly was palpated. CBC was abnormal for hemoglobin 8.1 g/dL, platelet 60,000/µL, leukocyte count 87000/µL with 64% blast (Table 1). Arterial blood gases were pH 7.402, PCO2 38.3 mmHg, and PO2 67 mmHg; oxygen saturation was 89% without supplemental oxygen.
Table 1. Complete blood count with differential.
Laboratory
studies | Results |
|---|
| Hemoglobin | 8.1 g/dL
(81 g/L) |
| Platelets | 60,000/µL
(60 × 109/L) |
| Leukocyte | 87,000/µL
(87 × 109/L) |
| Blast | 64% |
| Neutrophils | 10% |
| Lymphocytes | 14% |
A hematologic malignancy was suggestive due to the serum dyscrasia. Chest radiograph showed a large free flowing left pleural effusion (Figure 1). A diagnostic and therapeutic thoracentesis was performed with removal of approximately 1 liter of fluid. The symptoms resolved and biochemical analysis established an exudative etiology (Table 2). The cytopathology specimen obtained from the pleural fluid was positive for blast cells with 62% leukemic myeloblast. AML was confirmed by bone marrow biopsy with expression of the antigens CD 34+ and CD 13+ (Figure 2) with intermediate to unfavorable cytogenetic prognosis (Table 3).
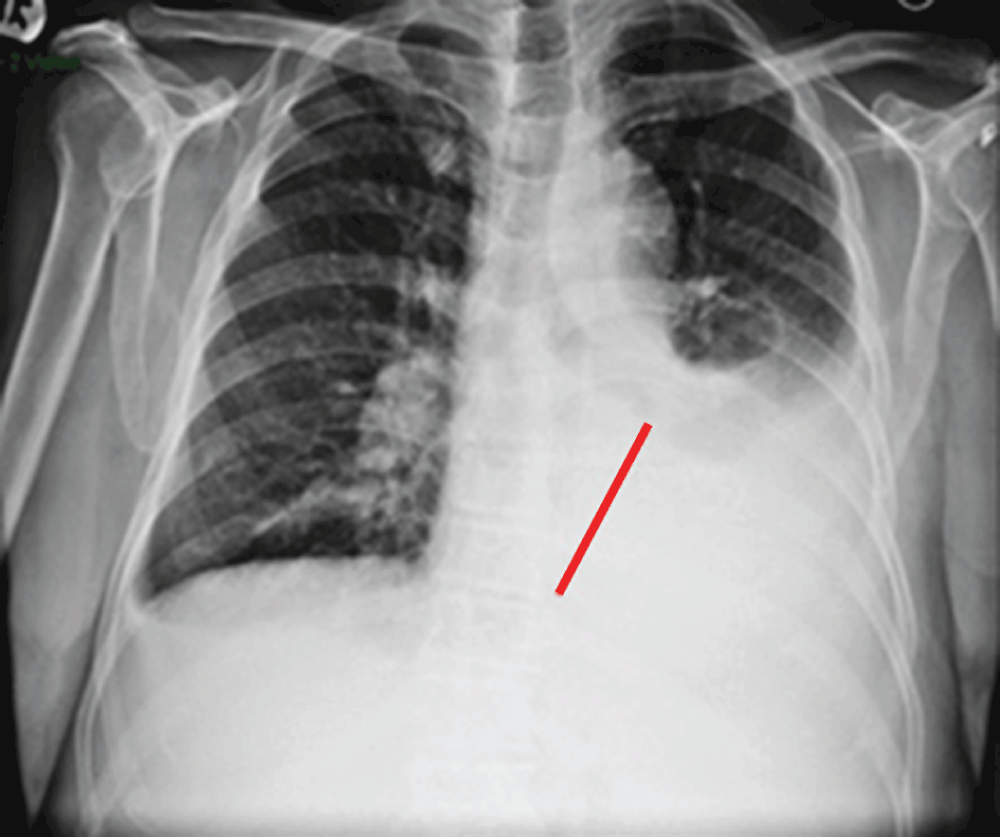
Figure 1. CXR with left pleural effusion (red bar).
Table 2. Pleural fluid description.
Ratio of the pleural fluid lactate dehydrogenase and protein to serum lactate dehydrogenase and protein (Light’s criteria meeting exudative etiology).
| Color | Protein | LDH | Glucose | pH | PFprotein/
Serumprotein | PFLDH/
SerumLDH |
|---|
| Dark yellow | 5.4 | 537 | 88 | 7.5 | 0.74 | 1.09 |
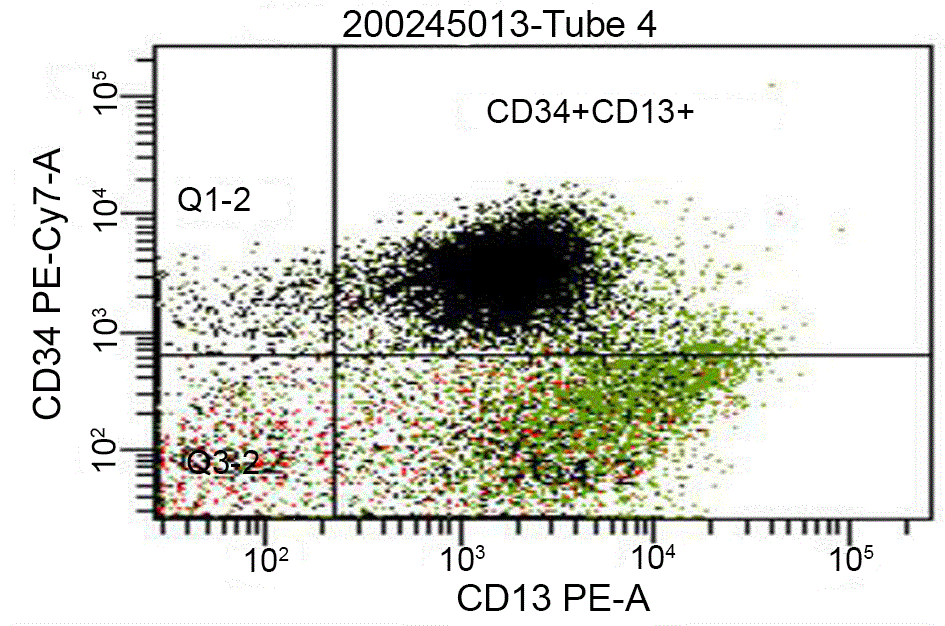
Figure 2. Flow cytometric quantification and immunophenotyping of leukemic stem cells in our patient with acute myeloid leukemia demonstrating expression of CD 34+ and CD13+ antigens on immature cells.
Table 3. Flow cytometry differential of leukocyte population demonstrating low immunophenotypic values of lymphocytes and granulocytes which demonstrates an unfavorable cytogenetic prognosis.
| Flow cytometry differential (% of Total cells) |
|---|
| Lymphocytes | 2 |
| B-cells | <1 |
| Kappa | <1 |
| Lambda | <1 |
| Kappa:Lamda Ratio | 1 |
| T-cells | 1 |
| CD4 | 1 |
| CD8 | 1 |
| CD4:CD8 Ratio | 1.6 |
| CD3+CD56+ | <1 |
| Natural killer cells | 1 |
| Monocytes | 7 |
| Granulocytes | 20 |
| CD34-Positive blasts | 62 |
| Plasma cells | <1 |
| Viability | 99 |
A karyotypic abnormality of Trisomy 21 was revealed through cytogenetic studies (Figure 3), which is the second most common chromosomal defect in AML. The patient was treated with Idarubicin combined with Cytarabine for the recently discovered AML and there was no re-accumulation of the pleural fluid. However, bone marrow aspiration was repeated to assess response to chemotherapy and he still presented with 62% of blasts cells. A new cycle of chemotherapy was started with Mitoxantrone, Etoposide and Cytarabine but only a partial response was obtained. Despite the therapeutic regimen, due to the severity of the disease and the poor cytogenetic prognosis, the patient’s condition deteriorated. In view of no significant response to therapy and dismal prognosis, supportive measures and palliative care was provided and eventually the patient died due to complications associated to AML.
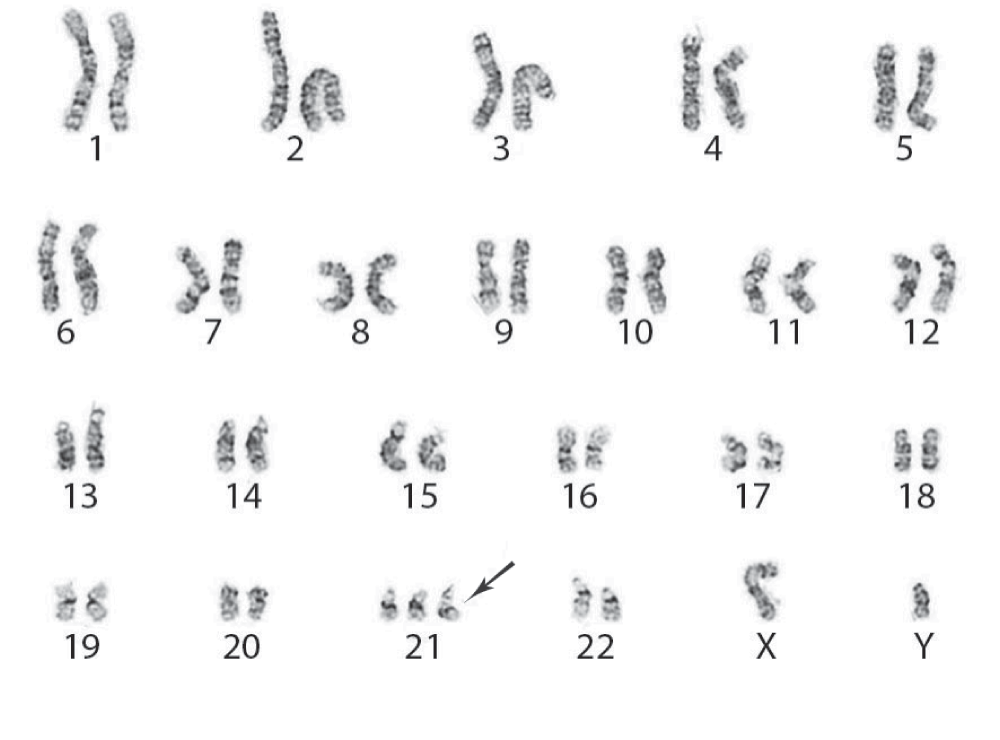
Figure 3. Trisomy 21 as the sole acquired karyotypic abnormality in our patient with acute myeloid leukemia (arrow).
Discussion
Physicians dealing with the diagnostic workup of pleural effusions rarely discover an underlying hematologic malignancy1. AML generally presents with symptoms related to complications of pancytopenia. Most patients have more subtle evidence of bone marrow involvement for weeks, or perhaps months, before the diagnosis can be made. Despite the pancytopenia, and/or coagulopathy, it is unusual for leukemias, either acute or chronic, to manifest with malignant pleural effusions as the initial presentation3–5. Usually this abnormal amount of fluid collection is a complication more commonly seen in solid tumors and lymphomas5.
Our patient presented with AML and pulmonary involvement with signs and symptoms secondary to the pleural effusion itself rather than with classical appearance of the acute myeloid leukemia. An unusual case where neither the complications of the hematologic dyscrasia such as bleeding and recurrent infections, nor the physical findings of a swollen spleen, liver, or lymph nodes were the primary target organs that would lead to a presumptive diagnosis. This demonstrates the importance of the biochemical analysis and the cytopathology specimens obtained in pleural fluid since an early detection of any determined disease could guide effective therapy6,7. AML in this particular case, and prompt treatment could undoubtedly contribute in avoiding complications associated with the condition; an essential factor for improving quality of life. For this reason chest physicians should be aware of all possible pulmonary manifestations of hematologic malignancies.
Consent
Written informed consent for publication of clinical details and clinical images was obtained from the relative of the patient.
Author contributions
Authors have contributed to the literature review, drafting of the manuscript, revisions of the manuscript and have agreed to the final content.
Competing interests
No competing interests have been disclosed.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Faculty Opinions recommendedReferences
- 1.
Jaffe ES, Harris NL, Stein H, et al.:
Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumours, Volume 3. Lyon IARC Press 2001. Reference Source
- 2.
Dores GM, Devesa SS, Curtis RE, et al.:
Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007.
Blood.
2012; 119(1): 34–43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Byrd JC, Edenfield WJ, Shields DJ, et al.:
Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review.
J Clin Oncol.
1995; 13(7): 1800–16. PubMed Abstract
- 4.
Ohe K, Okamura T, Arima F, et al.:
CD7 positive Acute Myelogenous Leukemia exhibiting pleural involvement as an initial manifestation.
Rinsho Ketsueki.
1994; 35(6): 552–556 [Article in Japanese]. PubMed Abstract
- 5.
Alexandrakis MG, Passam FH, Kyriakou DS, et al.:
Pleural Effusions in Hematologic Malignancies.
Chest.
2004; 125(4): 1546–1555. PubMed Abstract
| Publisher Full Text
- 6.
Wan TS, Au WY, Chan JC, et al.:
Trisomy 21 as the sole acquired karyotypic abnormality in acute myeloid leukemia and myelodysplastic syndrome.
Leuk Res.
1999; 23(11): 1079–83. PubMed Abstract
| Publisher Full Text
- 7.
Cortes JE, Kantarjian H, O’Brien S, et al.:
Clinical and prognostic significance of trisomy 21 in adult patients with Acute Myelogenous Leukemia and Myelodysplastic Syndromes.
Leukemia.
1995; 9(1): 115–7. PubMed Abstract



Comments on this article Comments (0)