Introduction
Salivary duct carcinoma is a distinctive primary neoplasm of the major salivary gland first described by Kleinsasser et al in 19681. The term was selected because of its resemblance to ductal carcinoma of the breast. It is characterized by aggressive behavior with early metastasis, local recurrence and significant mortality. Nearly 85% of cases occur in the parotid gland followed by submandibular gland2. The tumor has predilection for older men in the 6th to 7th decades of life3. A number of patients experience facial nerve palsy or paralysis and/or pain, and have cervical lymphadenopathy on presentation1. Familiarity with this entity is necessary to avoid false interpretation.
Case report
A 40 year old Hindu male who had a 15 year history of smoking presented with a gradually increasing painless swelling on the left parotid region. On examination an 8 × 6 cm swelling was observed the A single Level II mobile lymph node of size < 1 cm was palpable. There was no facial palsy.
An ultrasonograph of the parotid region performed previously revealed a well defined hypoechoic mass (4.7 × 3.9 cm) with lobulation occupying the left temporomandibular joint with adjacent hypoechoic areas of varying sizes: 1.9 × 1.6 cm, 1.4 × 1.5 cm, and 1.1 × 1.2 cm. It was interpreted as a parotid mass. Fine needle aspiration cytology (FNAC) from the left parotid gland was done and reported as a pleomorphic adenoma. However, FNAC from the submandibular lymph node comprised of blood only.
A repeat FNAC at our institute (Figure 1) showed moderately cellular aspirates with few clusters of normal salivary gland tissue along with epithelial cells showing overcrowding, with a mild-to-moderate pleomorphic population of medium sized cells with the vesicular nuclei having evenly distributed chromatin without conspicuous nucleoi. Cytoplasm was eosinophilic with ill defined borders. It was interpreted as an epithelial neoplasm.
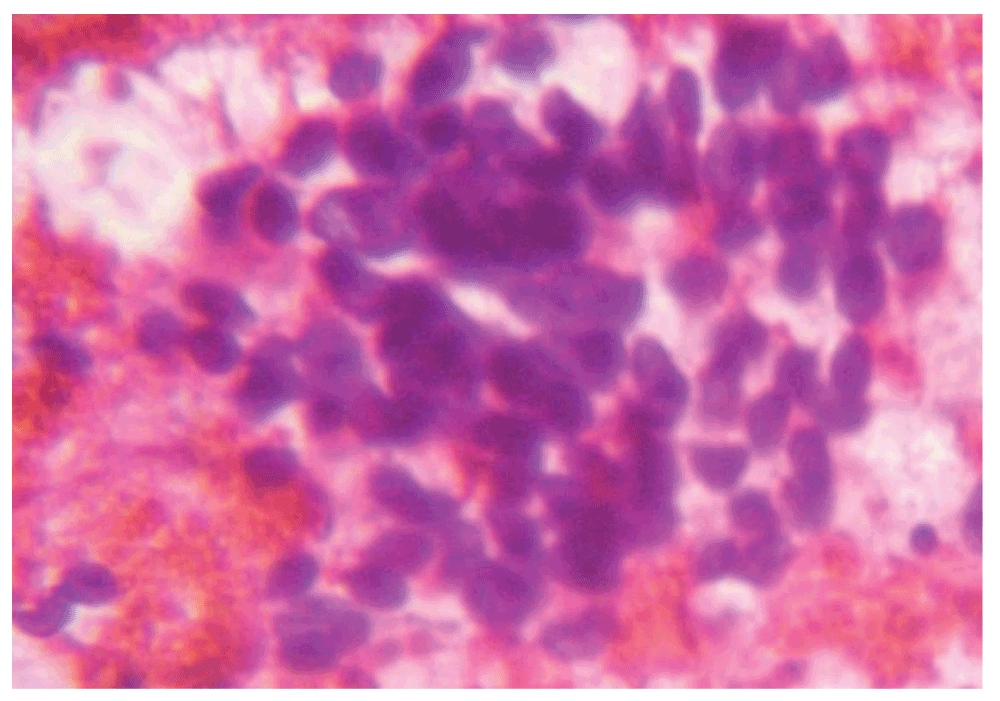
Figure 1. A hematoxylin and eosin stain of fine needle aspiration cytology of the parotid gland (40x).
A CT scan of the face and neck showed that the left parotid gland had enlarged in size (9 × 6 × 4 cm), involving deep and superficial lobes, with replacement of normal glandular architecture by homogenous soft tissue. The adjacent musculo-fascial planes were preserved. Multiple enlarged discrete lymph nodes in the left parotid were noted. The left internal jugular vein was compressed and no intraluminal thrombosis was seen.
In order to reach a diagnosis, a frozen Level II lymph node was performed. (Figure 2) On frozen section, the lymph node architecture was not seen. Cells were singly scattered having scanty cytoplasm, enlarged hyperchromatic nuclei and condensed chromatin. It was not possible to identify the type of malignancy and was therefore reported as a high grade malignant neoplasm.
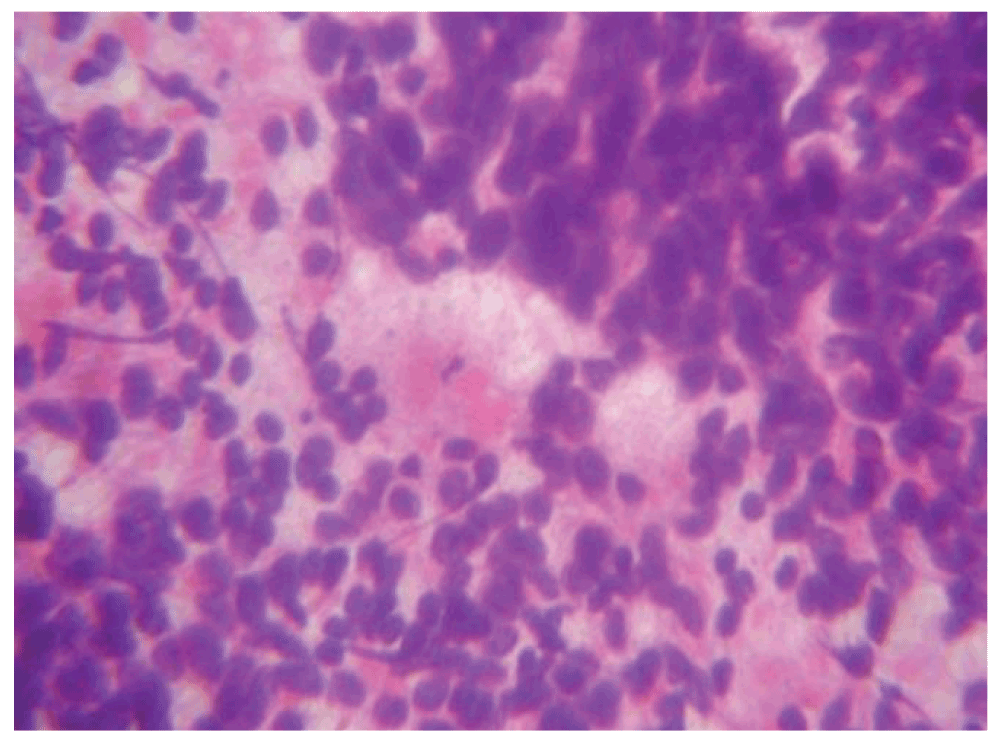
Figure 2. A hematoxylin and eosin stain of a frozen level III lymph node (40x).
On routine histopathology, neck nodes were resected. Six out of seven lymph nodes showed a metastatic neoplasm comprising of sheets and lobules of pleomorphic cells with coarse clumped chromatin separated by fibrous septa. Mitotic activity increased, rosette formation was noted, and it was reported as a poorly differentiated carcinoma with basaloid phenotype. The presence of a high mitotic rate and of focal large, polypoid nuclei suggested an origin from the sebaceous gland.
Finally, a total parotidectomy with modified neck dissection was performed. On gross examination the specimen comprised of:
– Single gray soft tissue piece with skin tag (15 × 14 × 4 cm).
– Skin (4.5 × 2 cm).
– Salivary glands (4 × 2 × 2 cm) were grossly unremarkable.
– Multiple lymph nodes at level II & III – (1.5 to 5.5 cm).
– A deep lobe parotid gland.
– Several gray soft tissue pieces (7 × 7 × 3 cm) with a level I lymph node.
– A single gray soft tissue piece (4 × 4 × 1 cm) (Figure 3).
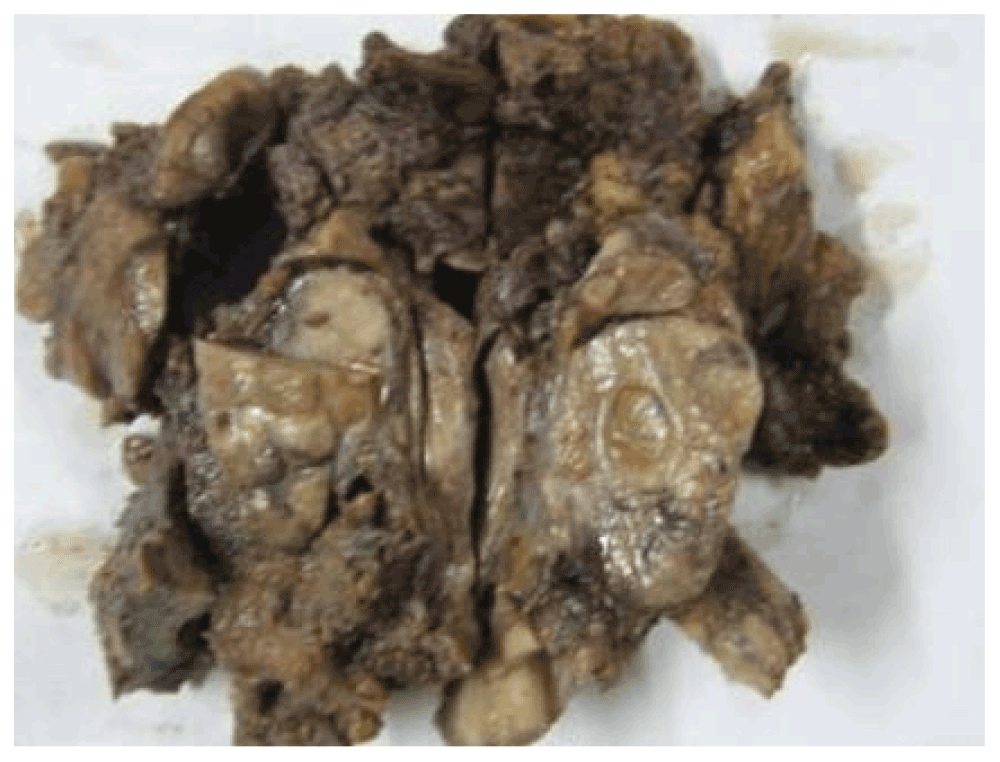
Figure 3. A gross photo of the parotidectomy.
Microscopic analysis (Figure 4, Figure 5) showed that the tumor comprised of slightly pleomorhic ovoid cells with vesicular nuclei arranged in sheets and a trabecular pattern separated by fibrous septa. Mitotic activity was not increased. Focal area showed an acinar and comedo pattern. Perineural and lymphovascular invasion were seen. Infiltration into the salivary gland tissue was noted. Eight out of ten lymph nodes showed metastatic carcinoma. In view of metastasis to a lymph node, a diagnosis of high grade malignant epithelial neoplasm was suggested, which was later confirmed via immunohistochemistry as salivary duct carcinoma.
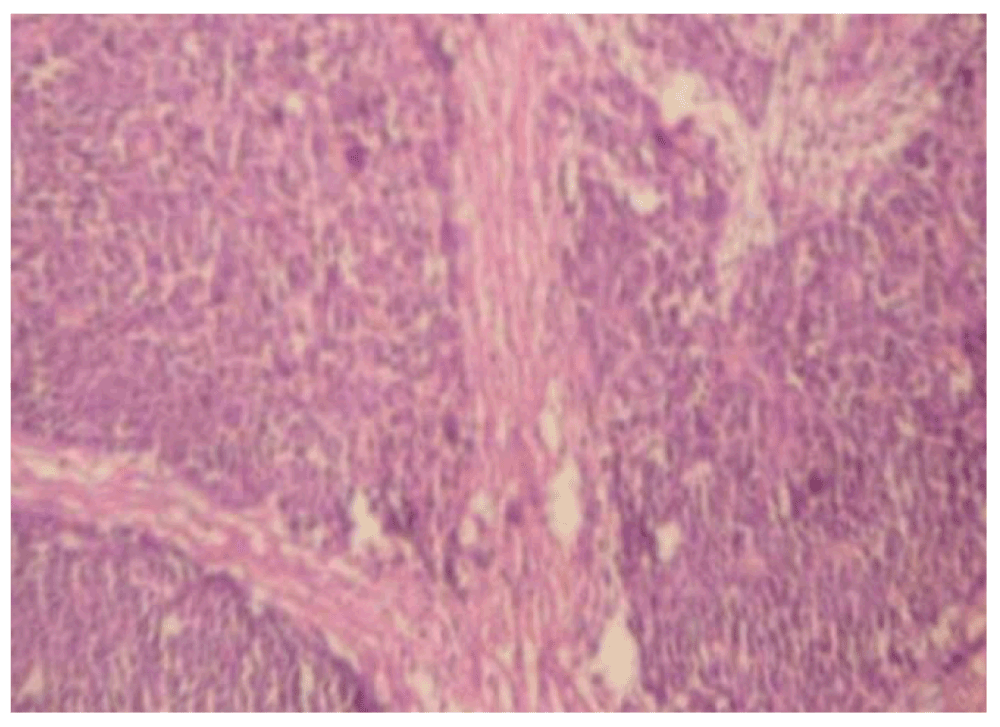
Figure 4. A hematoxylin and eosin stain of the parotid gland tumor (10x).
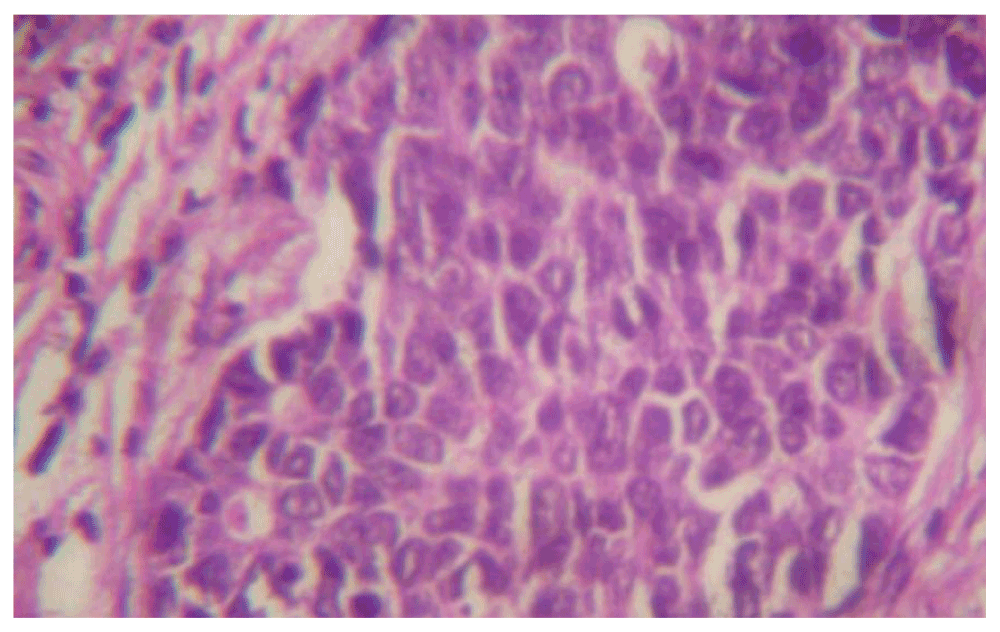
Figure 5. A hematoxylin and eosin stain of the parotid gland tumor (40x).
Discussion
Salivary duct carcinoma (SDC) is an aggressive adenocarcinoma which resembles high-grade breast ductal carcinoma. It is also known as cribriform salivary carcinoma of excretory ducts, or high-grade salivary duct carcinoma. SDC represents 9% of salivary malignancies. The male: female ratio is at least 4:1 and most patients present after age 504. The parotid is most commonly involved, but submandibular, sublingual, minor salivary gland, maxillary and laryngeal tumours have been reported2. SDCs are usually firm, solid, tan, white or grey, with a cystic component. Infiltration of the adjacent parenchyma is usually obvious, but occasional tumours may appear to be circumscribed. SDC may also arise as the malignant component of a carcinoma ex-pleomorphic adenoma, so that the macroscopic features of pleomorphic adenoma may also be present. For SDC, perineural spread (60%) and intravascular tumour emboli (31%) are common4. SDC resembles intraductal and infiltrating mammary duct carcinoma, both architecturally and cytologically. The diagnostic “ductal lesion” comprises pleomorphic, epithelioid tumour cells with a cribriform growth pattern, “Roman bridge” formation, and intraductal comedonecrosis. Cytologically, these cells have abundant, pink cytoplasm and large pleomorphic nuclei with prominent nucleoli and coarse chromatin. The cytoplasm may also be densely eosinophilic, granular, or oncocytic. Mitotic figures are usually abundant. Goblet cells are not seen5,6.
Immunohistochemistry
SDC is immunoreactive for low- and high-molecular-weight cytokeratin, and markers such as carcinoembryonic antigen (CEA), LeuM1, and epithelial membrane antigen (EMA)7. Strong nuclear reactivity for androgen receptors (AR) is reported in all SDC. As well as being positive for GCDFP-15, they are negative for S-100 protein, myoepithelial markers as well as estrogen and progesterone receptors8. The MIB1 proliferative index is high. Most SDCs show positive distinct membrane staining for HER-2/neu protein. Metastatic breast and squamous carcinomas, oncocytic carcinoma and mucoepidermoid carcinoma come in differential diagnosis because they also show similar immunohistochemistry profiles9–11.
SDC is one of the most aggressive salivary malignancies. Sites for distant metastasis include lungs, bones, liver, brain and skin4. Sixty-five percent of patients die from the disease, usually within 4 years of diagnosis (ranging from 5 months to 10 years)11. The clinical course is characterized by early distant metastases. Tumour size, distant metastasis, and HER-2/neu overexpression are putative prognostic parameters for SDC, while expression of p53 protein, DNA aneuploidy, and proliferative activity do not correlate with outcome9,10. The clinical outcome for the mucin-rich variant of SDC is similar to that of conventional SDC12.
Conclusion
Given the known difficulty in making an accurate diagnosis of salivary duct carcinoma13, the identification of a tumor exhibiting variable nuclear grade with cribriform, papillary and comedo patterns in the appropriate clinical setting of elderly patients with parotid mass and facial palsy should suggest the diagnosis of this uncommon tumor after excluding a metastatic carcinoma.
Consent
Written Informed consent for publication was obtained from the patient.
Author contributions
RS and CLP contributed to the conception and design of the study. RS collected and analyzed the data and wrote up the manuscript. RS and CLP both approved the manuscript.
Competing interests
No competing interests were disclosed.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Acknowledgements
Mr Mudit Sharma Senior Technician, Department of Pathology and Bhagwan Mahaveer Cancer Hospital and Research Center, Jaipur, India for assisting in performing section cutting, staining and special stains.
Faculty Opinions recommendedReferences
- 1.
Lewis JE, McKinney BC, Weiland LH, et al.:
Salivary Duct carcinoma. Clinicopathologic and Immunohistochemical Review of 26 Cases.
Cancer.
1996; 77(2): 223–30. PubMed Abstract
| Publisher Full Text
- 2.
Epivatianos A, Dimitrakopoulos J, Trigonidis G:
Intraoral salivary duct carcinoma: a clinicopathological study of four cases and review of the literature.
Ann Dent.
1995; 54(1–2): 36–40. PubMed Abstract
- 3.
Boson WL, Gomez RS, Araujo L, et al.:
Odontogenic myxomas are not associated with activating mutations of the Gs alpha gene.
Anticancer Res.
1998; 18(6A): 4415–4417. PubMed Abstract
- 4.
Barnes L, Rao U, Krause J, et al.:
Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature.
Oral Surg Oral Med Oral Pathol.
1994; 78(1): 64–73. PubMed Abstract
| Publisher Full Text
- 5.
Henley JD, Seo IS, Dayan D, et al.:
Sarcomatoid salivary duct carcinoma of the parotid gland.
Hum Pathol.
2000; 31(2): 208–213. PubMed Abstract
| Publisher Full Text
- 6.
Nagao T, Gaffey TA, Serizawa H, et al.:
Sarcomatoid variant of salivary duct carcinoma: clinicopathologic and immunohistochemical study of eight cases with review of the literature.
Am J Clin Pathol.
2004; 122(2): 222–231. PubMed Abstract
| Publisher Full Text
- 7.
Delgado R, Vuitch F, Albores-Saavedra J:
Salivary duct carcinoma.
Cancer.
1993; 72(5): 1503–1512. PubMed Abstract
| Publisher Full Text
- 8.
Skalova A, Starek I, Vanecek T, et al.:
Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry.
Histopathology.
2003; 42(4): 348–356. PubMed Abstract
| Publisher Full Text
- 9.
Kapadia SB, Barnes L:
Expression of androgen receptor, gross cystic disease fluid protein, and CD44 in salivary duct carcinoma.
Mod Pathol.
1998; 11(11): 1033–1038. PubMed Abstract
- 10.
Martinez-Barba E, Cortes-Guardiola JA, Minguela-Puras A, et al.:
Salivary duct carcinoma: clinicopathological and immunohistochemical studies.
J Craniomaxillofac Surg.
1997; 25(6): 328–334. PubMed Abstract
| Publisher Full Text
- 11.
Brandwein MS, Jagirdar J, Patil J, et al.:
Salivary duct carcinoma (cribriform salivary carcinoma of excretory ducts). A clinicopathologic and immunohistochemical study of 12 cases.
Cancer.
1990; 65(10): 2307–2314. PubMed Abstract
| Publisher Full Text
- 12.
Simpsonn RHW, Prasad AR, Lewis JE, et al.:
Mucin-rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four cases.
Am J Surg Pathol.
2003; 27(8): 1070–1079. PubMed Abstract
| Publisher Full Text
- 13.
Kinnera V, Nandyala R, Yootla M, et al.:
Salivary duct carcinoma of parotid gland.
Internet J Pathol.
2009; 10(1). Reference Source





Comments on this article Comments (0)