Introduction
Among the hundreds of distinct cell types that make up our bodies, neurons are the most morphologically complex, and also one of the most dynamic in their responsiveness and adaptability. Neurons contain structural specialisations, which allow the rapid processing and transmission of the thousands of synaptic inputs that they simultaneously receive. The vast majority of excitatory synapses involving the neurotransmitter glutamate are made into dendrites, which are extensions of the plasma membrane that can span hundreds of microns and cover broad fields in the tissue1. Release of glutamate into the synaptic cleft induces transient postsynaptic electrical and biochemical responses, which can eventually promote stable changes in the properties of the neuron2. At an excitatory synapse, glutamate acts on both metabotropic (mGluRs) and the ionotropic N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors3,4. mGluRs are G-protein coupled receptors (GPCRs) that are linked to heterotrimeric G proteins on the intracellular side of the membrane. Activation of mGluRs by glutamate modulates the activity of various signal transduction pathways through the change in concentration of intracellular second messengers such as intracellular inositol 1,4,5-trisphosphate (IP3) and cyclic adenosine monophosphate (cAMP), which controls the cAMP-dependent protein kinase (PKA)5. Glutamate binding to AMPA receptors (AMPARs) opens the ion channel and induces fast depolarisation of the postsynaptic membrane, mainly through the influx of sodium (Na+) ions. By contrast, glutamate-gated channel opening of NMDA receptors enables calcium (Ca2+) influx into the dendritic spine, which initiates a cascade of signalling events involving the stimulation of the Ca2+/calmodulin-dependent protein kinase (CaMK) as well as the extracellular signal regulated kinase (ERK). The stimulation of CaMK and ERK triggers the phosphorylation-induced activation of a myriad of cellular targets including ion channels and transmembrane receptors, which in turn modifies their conductance properties6,7.
Additionally, an important consequence of the activation of these signalling pathways upon excitatory neurotransmission is that they can regulate the activity of nuclear factors thereby triggering changes in gene transcription8. The mechanism by which neuronal activity is conveyed to the nucleus for the induction of activity-dependent gene expression programs is one of the most investigated topics in neuroscience, since it is believed to be necessary for the establishment of memories. Indeed, mRNA synthesis inhibitors such as actinomycin D have been shown to prevent late long-term potentiation in hippocampal slices and to impair retention of new memories in several species and learning paradigms9–13. Interestingly, former studies regarding activity-dependent regulation of transcription showed that induction of genes occurs within a few minutes after excitatory electrical and pharmacological stimulation14–17. These studies demonstrate that, in spite of the highly polarised morphology of neurons, synaptic signals are rapidly conveyed to the nucleus to allow the immediate regulation of gene transcription.
Various mechanisms have been proposed that might couple synaptic activity to gene transcription18–20. The main difference among these models relies on the nature of the signal that carries the message, i.e. synapto-nuclear messengers, IP3-triggered Ca2+ waves or action potentials. This review will critically discuss the primary experimental findings supporting the current models for communication between synapses and the nucleus and ascertain their potential role in efficiently and timely coupling neuronal activity to gene expression.
Translocation of transcription regulators from synapses to the nucleus
The restriction of effector proteins to particular subcellular locations is a commonly employed strategy used by most known signal transduction cascades, and allows coordination of signalling events in space and time21–23. In many cases, the activation of signalling proteins by upstream regulators, or their activation of downstream effectors, involves their rapid and regulated translocation to specific subcellular compartments24. Indeed, nuclear translocation of signalling molecules is known to play a role in the timely expression of genes in response to extracellular stimuli25–27. For example, although PKA and ERK are preferentially distributed in the cytoplasm in resting conditions, the rise of intracellular second messengers drives their rapid accumulation in the nucleus, where they phosphorylate multiple nuclear targets28,29. Furthermore, transcription factors can also relocate from the cytoplasm to the nucleus in response to stimuli that induce apoptosis, cell differentiation or proliferation30–32. There are several advantages of moving signalling proteins between neighbouring subcellular compartments upon cellular activity, including the enhancement of the specificity as well as speed of signal transmission22–34. Interestingly, there is strong evidence demonstrating that a variety of stimuli mimicking neuronal activity, such as bath application of neurotransmitters or electrical stimulation, also induce the cyto-nuclear translocation of signalling proteins in primary neuronal cultures and brain slice preparations35,36. Moreover, the robust accumulation of gene transcription regulators in the nucleus is also observed in response to a wide variety of physiological and pharmacological stimuli in vivo. For example, immunohistological analysis of mouse brain sections have shown that the rapid nuclear accumulation of activated ERK in various regions of the brain including the hippocampus, amygdala and projection neurons of the striatum, occurs in animals that have been trained to behavioural paradigms that underlie learning, or following acute administration of addictive and non-addictive drugs37–41.
Synapse-to-nucleus protein translocation
Although the translocation of proteins has been demonstrated to occur between adjacent subcellular compartments i.e. from the cytoplasm to the plasma membrane or from the cytoplasm to the nucleus, it has been hypothesised that it can also occur between more distant compartments, linking dendritic terminals and axon tips with the nucleus42,43. Indeed, the retrograde transport of signals from distal axons to neuronal cell bodies by motor-dependent transport along microtubules is one of the best known mechanisms for long-distance communication44. So far, these mechanisms have been studied in the context of development, survival and axonal injury45. However, recent studies have proposed that activity at excitatory synapses can also promote the movement of proteins from distant parts of the dendritic arbour to the nucleus (Reviewed in18,20,46). This idea of long distance rather than local signalling in response to synaptic inputs provides a tantalising model in which the transport of transcription regulators from stimulated synapses serves to inform the genome about peripheral neuronal activity (Figure 1).
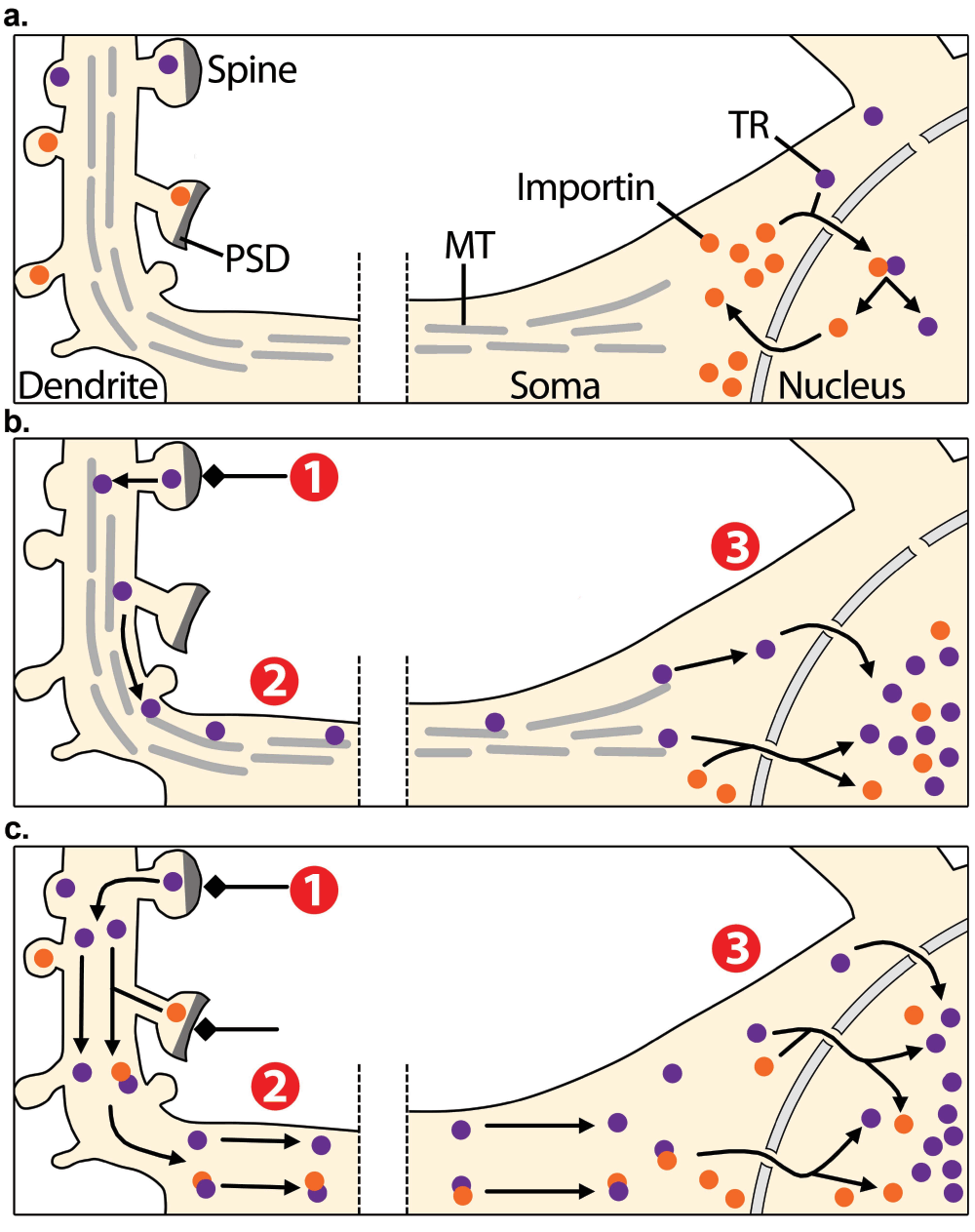
Figure 1. Synapto-nuclear translocation of transcription regulators.
a) In non-stimulated conditions, transcription regulators (TR, purple dots) are localised into distant dendrites as well as at the perinuclear zone. Some TRs are transported from the cytoplasm to the nucleus by importins (orange dots). Importins are also distributed across distant dendrites and at the postsynaptic density (PSD). b, c) Excitatory inputs that stimulate synapses (1) are believed to activate TRs, which are then transported to the soma along the neuron (2) through microtubule-based active transport b) or by passive diffusion c). Excitatory inputs are also proposed to activate importins at the PSD, which then associate to synaptic cargoes and facilitate their synapto-nuclear translocation c). Therefore, this model predicts that synaptic activity triggers the accumulation of TRs from distant synapses in the nucleus, promoting the induction of gene expression programs (3).
Former experimental evidence supporting this hypothesis comes from early studies of brain cell fractionation that were published by different laboratories in the 1990s43,47,48. Intriguingly, it was reported that the nuclear factor κ light chain enhancer of activated B-cells (NF-κB) can be detected in the synaptosomal fraction of cortical and hippocampal preparations, which contains pre- and post-synaptic elements of neurons. Additional subsequent studies have later shown that other transcription factors are also located at synaptosome preparations, including the cAMP response element-binding protein (CREB)49, CREB250 and CREB-regulated transcription coactivator 1 (CRTC1)51. Additionally, fluorescence microscopy analyses of endogenous proteins or fluorescently tagged chimeric constructs conducted to determine the subcellular localisation of these transcription regulators in neurons indicate that they are indeed not confined to the vicinity of the nucleus, but rather extend throughout the whole cytoplasm, reaching distant compartments like dendritic spines. Provided that the only recognised function of these transcription factors takes place in the nucleus where their known cellular targets belong, the intriguing question of how they can be efficiently and timely shuttled to the nucleus to influence transcription arises naturally.
Martin and colleagues have proposed a mechanism for the shipping of activated transcription regulators between synapses and the nucleus52,53. It involves the participation of members of the karyopherin family of nuclear transport receptors, which are soluble carriers that mediate the regulated translocation of proteins across the nuclear envelope54,55. The active import of proteins from the cytoplasm to the nucleus is mostly driven by a heterodimeric carrier composed of importin-β and its adaptor importin-α. Importins discriminate their cargo from other cellular proteins by recognition of amino acid targeting sequences known as nuclear localisation signals (NLSs), which contain one or two clusters of basic residues. Monopartite NLSs, exemplified by the SV40 large-T antigen56, have a single cluster of 4–5 basic residues (e.g. P KKKRKV), whereas bipartite NLSs, such as that of nucleoplasmin57, have a second basic cluster located 10–12 residues downstream from the first cluster (e.g. KRPAATKKAGOA KKKLD). Interestingly, immunofluorescence studies have detected importins far away from the nucleus, including dendrites and the axon of mouse hippocampal and Aplysia sensory neurons, suggesting a role in long-distance trafficking of proteins towards the nucleus52. Furthermore, strong accumulation of importins in the nucleus is observed in response to stimuli that mimic synaptic activity in neuronal primary cultures and hippocampal slice preparations. These observations led to the hypothesis that importins may direct the translocation of synaptic proteins to the nucleus53. In this line, a study by Holmes and co-workers reported that the cytoplasmic tail of the NR1-1a subunit of the NDMAR contains a putative bipartite NLS which is surrounded by four phosphorylation sites i.e. PDP KKKATFRAITSTLASSF KRRRSSKDT58,59. Based on these results, subsequent co-immunoprecipitation experiments by Jeffrey and colleagues showed that importin-α binds to NR1 in vitro, and that this interaction is disrupted by the activity-dependent phosphorylation of the residues flanking the NLS of NR1-1a60. The authors presented a model in which importins are normally tethered to the NLS of the NMDAR in non-stimulated synapses, placing them in close proximity to synaptically localised transcription regulators. Upon stimulation, phosphorylation of the NR1-1a tail by PKA and protein kinase C (PKC) promotes the release of bound importins that are then free to bind soluble synaptic NLS-containing cargoes such as the transcription factor CREB250. In view of this appealing mechanism for the long-range transport of proteins to the nucleus, a series of proteomic and bioinformatic studies have been conducted in order to identify novel NLS-containing proteins undergoing importin-mediated synapto-nuclear translocation61. Surprisingly, around 166 out of 1100 proteins from postsynaptic density (PSD) purified fractions were predicted to contain putative NLSs62. Among them, there were found several proteins involved in RNA trafficking and splicing, as well as regulators of transcription factor activity61,63.
Limitations and challenges to the model
Although a number of arguments support the existence of synapto-nuclear protein messengers, there are many remaining concerns that challenge the synapto-nuclear translocation model. One important caveat to be considered regards the identification of potential synapto-nuclear messengers by mass spectrometry. It has been observed that due to the high sensitivity of protein identification by this method, low-level contaminants introduced during the biochemical purification of brain fractions are commonly detected64. Moreover, searching for synapto-nuclear messengers by NLS identification may yield false positive results61, since similar clusters of basic residues are present in kinase and phosphatase docking sites and help to mediate protein-protein interaction65. For example, the consensus phosphorylation site for PKC is R/K1–3, X2–0-S/T whereas for PKA, it is R-R-X-S/T-Φ, where X is any amino acid and Φ is a hydrophobic residue which closely resembles the above mentioned NLS found in the NR1-1a subunit of the NMDA receptor66. Accordingly, a detailed functional dissection of these motifs needs to be performed before concluding that they represent bona fide NLSs. Experiments tracking fluorescently tagged proteins through live-cell imaging techniques as well as the use of nuclear import assays in permeabilised cells should be routinely used in order to confirm the possible nuclear translocation of these proteins67–69.
Another point that needs to be directly demonstrated is whether transcription regulators distributed at distant parts of the neuron actually shuttle to the nucleus. Recent experiments using cultured hippocampal neurons that express a photoconvertible fluorescent form of the synapto-nuclear transcription factor CRTC1 indeed suggest that activation of distant dendrites by local glutamate uncaging can promote its accumulation to the nucleus51. However, the movement of single synaptic messengers on route to the nucleus has never been directly tracked, and therefore this point remains to be clarified. The combination of super-resolution fluorescence microscopy with live-cell single-molecule-based imaging may provide new insights into the dynamics of the long-range transport of proteins in neurons70.
An important question regarding the long-range transport of proteins to the nucleus concerns the speed at which these messengers may travel towards the soma, which is crucial to explain their potential involvement in timely coupling of neuronal activation to the robust induction of gene programs71. It has been estimated that a transcription factor involved in the rapid induction of activity-dependent genes should travel at nearly 75 microns per minute towards the nucleus, but NF-κB has been calculated to move at only 2 microns per minute72. It is currently unknown whether such a rapid movement of proteins from synapses to the nucleus may occur by diffusion, facilitated transport, signalling endosomes or whether it requires association with the microtubule network. Moreover, a significant limitation of long-range signalling by the synapto-nuclear shuttling of transcription factors is that the signal is not amplified nor regenerated on its way towards the nucleus. Distantly stimulated messengers are likely to be inactivated or lost in transit, thereby compromising the direct involvement of synaptic activity on the control of nuclear functions. For example, if a transcription factor is activated by phosphorylation at the synapse, protein phosphatase activity will likely cause its inactivation on its way to the nucleus. Finally, a critical issue that needs to be addressed is to determine the advantage, if any, of mobilising transcription regulators from distant synapses over those already located adjacent to the nucleus to control gene expression.
Despite the list of synapto-nuclear messengers is rapidly growing, there are considerable ambiguities in the synapse-to-nucleus translocation model that need to be clarified. Further research is essential to gain a more accurate understanding of the presumed role of these signalling mechanisms on the coupling of excitatory synaptic transmission to gene induction during neuronal plasticity.
Propagation of calcium waves from synapses to the nucleus
It is now well documented that the rise of Ca2+ concentration in the nucleus represents an essential step for the activity-dependent induction of gene expression programs19,73–75. Cytoplasmic Ca2+ concentration can increase via voltage- and ligand-gated channels as well as by release from intracellular Ca2+ stores76. The main internal Ca2+ store is the endoplasmic reticulum (ER), which acts both as a sink for Ca2+ that enters from the extracellular space, and as a source for Ca2+ release into the cytosol. The ER forms a continuous vesiculo-tubular system that constitutes an elaborated network distributed throughout the cytoplasm77. In neurons, this network extends from the outer nuclear envelope in the soma into axonal processes and dendritic arborisations, including spines78,79. Release of Ca2+ from the ER can occur upon increase of intracellular IP3 and adenosine diphosphate ribose (ADP-ribose), which bind to and open IP3 receptors (IP3Rs) and ryanodine-type receptors (RyRs), respectively80. As mentioned above, IP3 can be generated by the stimulation of mGluRs which can be coupled to phospholipase C (PLC), the enzyme that cleaves the membrane phospholipid phosphatidylinositol 4,5-bisphosphate to form IP381,82. Moreover, Ca2+ itself activates the opening of RyRs and enhances the Ca2+-releasing action of IP3, therefore stimulating the efflux of more Ca2+ from the ER lumen83. This process of Ca2+-induced Ca2+ release (CICR) is believed to establish regenerative Ca2+ waves that spread bidirectionally from their initiation site and was first discovered in skeletal muscle84,85. Indeed, CICR is considered to be the physiological mechanism of Ca2+ release in cardiac muscle, playing a major role in cardiac excitation-contraction coupling86.
The development of fluorescent Ca2+ indicator molecules, photolysis techniques and laser-scanning microscopy has enabled the investigation of the Ca2+ dynamics in neurons at very fine temporal and spatial resolutions87–90. Synaptically activated, IP3-mediated propagation of Ca2+ release has been observed in pyramidal neurons in the rodent hippocampus (CA1 and CA3 regions), medial prefrontal cortex and principal neurons in the amygdala91–95. Depending on the neuronal cell type analysed and the stimulation protocol used, dendritic Ca2+ waves can be highly localised or can spread to a larger extent96. For example, when IP3 is generated by focal synaptic stimulation with metabotropic agonists, the generated response is weak and restrained97–99. By contrast, when IP3 production is stimulated by bath application of such agonists, a higher response can be evoked and a rise in Ca2+ concentration can be observed in the nucleus99,100.
These observations have led to the hypothesis that excitatory inputs trigger IP3R-dependent CICR waves that are able to spread forward from the distant synapses towards the soma and mediate the release of Ca2+ from the inner membrane of the nuclear envelope into the nucleus (Figure 2)76. Therefore, it has been suggested that the process of CICR may serve to communicate between distant cellular compartments in neurons and serve as a means to mediate the coupling of synaptic activity to gene transcription. While this model has the advantage over the synapto-nuclear translocation model of being a regenerative process in which the synaptic signal does not decay over great distances, evidence for the distant communication by this mechanism is controversial. First, it is not well established whether functional Ca2+ channels are present in the nuclear envelope in neurons, and if they can indeed release Ca2+ into the nucleoplasm101–103. More detailed studies on the spatial distribution of receptors involved in Ca2+ release from the ER in neurons may resolve such discrepancies. Second, it is important to note that whereas Ca2+ alone is sufficient to cause its own release through RyRs104–106, it cannot do so through IP3Rs. In the case of IP3Rs, Ca2+ can cause self-release only in the presence of IP3, thus the availability of IP3 in the cytoplasm is a major factor determining the extent of Ca2+ wave propagation107–109. Because of these findings, CICR is generally considered as an exclusive property of RyRs, but not of IP3Rs, even though IP3R exhibits the apparent CICR behavior in the presence of IP386,110,111.
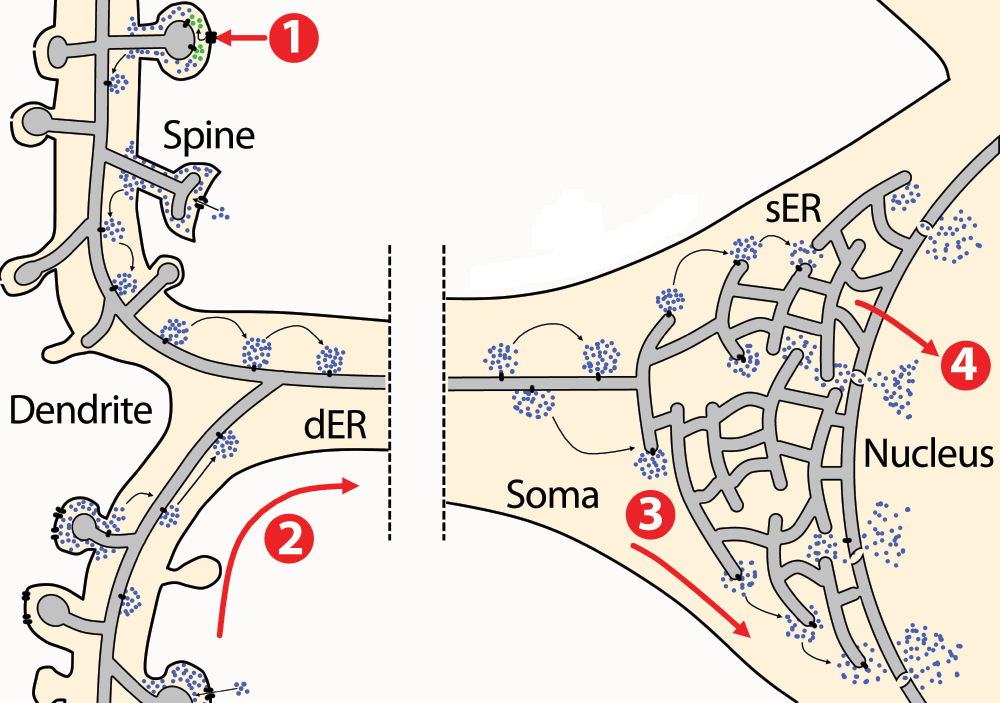
Figure 2. Calcium signal propagation from the synapse to the nucleus.
The endoplasmic reticulum (ER) is distributed throughout the cytoplasm from the nuclear envelope to dendritic spines. Excitatory synaptic stimulation through glutamate causes membrane depolarisation and entry of Ca2+ (blue dots) from the extracellular space through NMDARs (1). Moreover, glutamate also activates mGluRs coupled to PLC thereby stimulating IP3 production (green dots). Ca2+ and IP3 stimulate receptors present at the ER membrane that open and release more Ca2+ from the ER lumen, establishing a Ca2+-induced Ca2+-release wave (2) that propagates from dendrites towards the soma. In the soma, Ca2+ release from the ER activates cytoplasmic Ca2+-dependent signalling cascades that convey the signal to the nucleus (3). Moreover, Ca2+ can be released to the nucleus from the ER, where it activates nuclear transcription regulators (4).
As noted above, experiments in different types of neurons suggest that the spread of IP3-mediated Ca2+ waves depends on the availability of IP3 in the preparation96. Although IP3 has a fast diffusion constant, it has a limited spatial range of action as it is rapidly metabolised by 3-kinase and 5'-phosphomonoesterase and it is estimated to have a lifetime of a few seconds112,113. Moreover, during the past few years it has become clear that there are functional compartments existing within dendritic trees that control the diffusion of intracellular messengers, particularly within spines87,114. Hence, the restriction of mobilised IP3 to sites close to activated synapses makes it unlikely that synaptically triggered IP3-dependent CICRs spread to the soma and transport a message from the synapse. However, functional metabotropic receptors coupled to IP3 production are present at the level of the soma in the plasma and the inner nuclear membranes of several neuron types, raising the possibility that local rather than remotely generated IP3-dependent release of Ca2+ from the ER may contribute to the induction of gene transcription115–118.
Propagation of electrical signals along the plasma membrane
The models discussed in the previous sections are built on the assumption that activation of postsynaptic glutamate receptors triggers the transmission of intracellular messengers (i.e. transcription regulators and Ca2+ waves) from distant dendritic sites to the nucleus. However, several lines of evidence have suggested that gene expression can be triggered by NMDAR and mGluR-independent mechanisms119,120. Experiments using dorsal root ganglion cell culture preparations, which are devoid of synapses, demonstrated that phosphorylation of nuclear factors and induction of genes could be induced with electrical stimulation121. Furthermore, electrophysiological studies using hippocampal slice preparations have shown that somatic action potentials can trigger gene transcription without the involvement of synaptic activation and in the presence of NMDAR antagonists119,120,122. However, NMDARs appeared to have a role in the activation of nuclear events through their contribution to action potential generation122. These results led to a third model proposed by Dudek and colleagues, which posits that the robust depolarisation of the somatic plasma membrane is sufficient to induce gene transcription without the involvement of transported biochemical signals from distant synapses71,72,123. According to this model, transmission of synaptic events from distant compartments to the soma is achieved through the propagation of electrical signals over long distances across the plasma membrane. There are several mechanisms of long-range electrical propagation that regulate the degree of depolarisation at the soma. A simplified explanation of the process is briefly provided here, however the reader is referred to the excellent available reports fully addressing this topic124,125. On one hand, excitatory synaptic events lead to changes in the membrane conductance that can be forward-propagated to the soma in the form of dendritic spikes126,127. On the other hand, action potentials initiated at the axon initial segment can be retrogradely propagated into the soma and the dendritic tree, spreading back the electrical signal128. The range of propagation efficacies of dendritic spikes and backpropagated action potentials varies in different cell types and is influenced by multiple passive and active properties of the somatodendritic compartment, including dendritic geometry, voltage-gated channel densities and the spatial and temporal profile of synaptic excitation and inhibition125,129,130.
It is well established that membrane depolarisation in the soma causes the opening of voltage-gated Ca2+ channels (VGCCs), which allow the influx of Ca2+ from the extracellular space to the cytoplasm, thus coupling synaptic activity to intracellular signalling14,131. Interestingly, it appears that, among the different classes of VGCCs, L-type VGCCs seem to be significantly involved in regulating transcription since activity-dependent induction of genes is supressed by exposure to specific antagonists and increased by (-)BayK-8644, a VGCC agonist14,132,133. The differential ability of L-type VGCCs to preferentially convey signals to the nucleus over the other types of VGCCs may be due to their relatively slow inactivation rate, high single-channel conductance for Ca2+ and relative localisation to Ca2+ intracellular sinks134,135. In addition, Ca2+ entry through L-type VGCCs has been suggested to trigger ER-directed Ca2+ waves that propagate from the plasma membrane of the soma to the nucleus without the involvement of cyto-nuclear translocation of proteins73,76. These findings suggest that L-type VGCCs at the soma are well suited for coupling synaptic excitation to activation of transcriptional events.
It is important to note that the degree of membrane depolarisation, and thus the opening of L-type VGCCs, is influenced by the immediate electrical history of the neuron. Because postsynaptic events to individual synaptic inputs are usually small and transient, action potential initiation and dendritic spikes require strong synchronous synaptic activation to be generated124. Moreover, inhibitory inputs also affect the way in which excitatory synaptic inputs summate in space and time. Thus, the elaborate integration of excitatory and inhibitory inputs will govern the opening of VGCCs, thereby shaping the activation of Ca2+-dependent nuclear functions125. Taken together, the model upheld by Dudek and colleagues provides a cellular mechanism by which synaptic activity is coupled to gene transcription through VGCCs, which transduce electrical signals into biochemical pathways (Figure 3). Through this mechanism, signalling molecules are only required to translocate between neighbouring subcellular compartments, as observed in many other cell types, conveying information timely and efficiently.
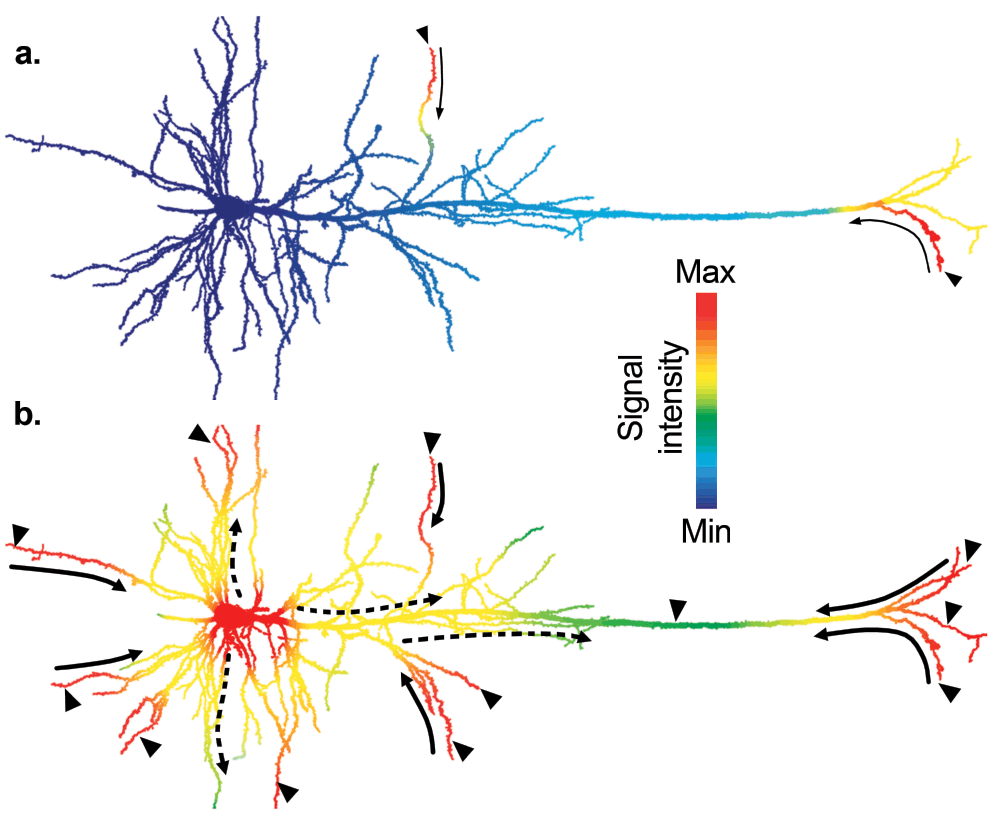
Figure 3. Activation of nuclear functions by action potentials.
Excitatory synaptic activity (arrowheads) generates local changes in membrane conductance, which causes the opening of voltage gated calcium channels (VGCCs). A rise in intracellular Ca2+ triggers the local activation of Ca2+-dependent signalling pathways thereby, coupling membrane depolarisation to intracellular signalling. a) When neurons receive weak excitatory inputs (small arrowheads), the signal spread is small (thin solid arrows) and the threshold to trigger action potentials is not reached, thus somatic L-VGCCs remain closed. b) When neurons receive strong synaptic inputs (big arrowheads), dendritic spikes can efficiently spread (thick solid arrows) in the forward direction and facilitate the initiation of action potentials at the axonal initial segment. Action potentials are backpropagated to the soma and dendrites (dashed arrows), locally generate intracellular Ca2+-dependent signalling cascades. Ca2+ entry in the soma through L-VGCCs will promote the activation of protein effectors that regulate the transcription of plasticity-related genes. In this diagram, the local signal intensity representing both the electrical activity of the membrane and its coupled intracellular signalling is coded by colour. Drawings are adapted from a reconstructed biocytin-filled layer V neuron in the rat cortex (courtesy of B. Chieng and J. Bertran-Gonzalez).
Importantly, this model predicts that action potentials will fail to induce gene transcription programs unless firing above a critical threshold, suggesting that intracellular mechanisms must exist to assess the degree of synaptic stimulation123. Knowledge of how the nucleus computes action potentials to promote gene transcription while avoiding background stimulation is therefore fundamental for understanding how synaptic inputs regulate activity-dependent gene transcription and neuronal plasticity.
Conclusion
Although it has been known for more than 25 years that gene induction upon excitatory transmission is a required step for neuronal plasticity, it is not yet clear whether a direct link between local synaptic activation and gene transcription exists. An increasing body of literature suggests that there are at least three different pathways by which neurons may inform the nucleus about the events happening at distant dendritic compartments18–20. In addition to the unique feature of neurons of communicating messages over long distances by electrical signals, it has been recently proposed that the long-range physical translocation of proteins from remote dendritic sites towards the soma can convey information to the nucleus20,46. The current evidence for the synapto-nuclear translocation model shows that transcription regulators, which were previously considered to be largely localised in close proximity to the nucleus, may also be present at remote sites from the nucleus, in postsynaptic dendritic compartments. Moreover, different stimuli that promote neuronal excitation trigger the accumulation of these gene transcription regulators in the nucleus, although it is still unclear whether they are the same ones activated at distant parts of the neuron. Several critical questions remain enigmatic, as to how and how fast the synapto-nuclear messengers are shipped to the nucleus, and what their actual roles are on gene induction. Taken together, and given the experimental data now available, it is very unlikely that this mechanism participates significantly in the activity-dependent regulation of gene transcription. Future experimental evidence would help to clarify their involvement in other aspects of neuronal physiology.
The most plausible scenario for relaying synaptic activity to the nucleus involves the robust propagation of action potentials, which are initiated upon integration of excitatory, inhibitory and modulatory inputs123. Influx of Ca2+ from the extracellular space through ligand-gated receptors (e.g. NMDAR) and VGCCs triggers intracellular signalling cascades that remain spatially confined and act locally to regulate cellular processes136. In the soma, the opening of L-type VGCCs causes a rise in intracellular Ca2+ and thereby activates Ca2+-dependent signalling molecules that shuttle from the cytoplasm to the nucleus such as activated ERK and NF-κB74. Moreover, it may also allow the generation of RyR- or IP3R-dependent Ca2+ waves that propagate along the ER from the plasma membrane to the nucleus inducing the increase of Ca2+ concentration within the nucleus76. In addition, it is anticipated that Ca2+-transduction pathways crosstalk with simultaneously activated signalling cascades governed by other second messengers such as IP3 and cAMP that will promote or restrain the spread of the Ca2+ signal and will have a profound influence on the precise timing and extent of gene induction. Future research will need to determine how these coordinated processes contribute to shape neuronal plasticity.
Competing interests
No competing interests were disclosed.
Grant information
M. Matamales is a recipient of an EMBO Long-Term Fellowship (ALTF 1228–2010).
Acknowledgements
The author would like to thank Dr. J. Bertran-Gonzalez and Dr. M. Cavazzini for their valuable comments on the manuscript.
Faculty Opinions recommendedReferences
- 1.
Fiala JC, Spacek J, Harris KM, et al.:
Dendrite structure.Dendrites.
Oxford University Press 2nd edition
2008.
- 2.
Flavell SW, Greenberg ME:
Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system.
Annu Rev Neurosci.
2008; 31: 563–90. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Nakanishi S:
Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity.
Neuron.
1994; 13: 1031–7. PubMed Abstract
| Publisher Full Text
- 4.
Nakanishi S, Nakajima Y, Masu M, et al.:
Glutamate receptors: brain function and signal transduction.
Brain Res Rev.
1998; 26: 230–235. PubMed Abstract
| Publisher Full Text
- 5.
Conn PJ, Pin JP:
Pharmacology and functions of metabotropic glutamate receptors.
Annu Rev Pharmacol Toxicol.
1997; 37: 205–37. PubMed Abstract
| Publisher Full Text
- 6.
Lisman J, Schulman H, Cline H:
The molecular basis of CaMKII function in synaptic and behavioural memory.
Nat Rev Neurosci.
2002; 3: 175–90. PubMed Abstract
| Publisher Full Text
- 7.
Adams JP, Sweatt JD:
Molecular psychology: roles for the ERK MAP kinase cascade in memory.
Annu Rev Pharmacol Toxicol.
2002; 42: 135–63. PubMed Abstract
| Publisher Full Text
- 8.
West AE, Greenberg ME:
Neuronal activity-regulated gene transcription in synapse development and cognitive function.
Cold Spring Harb Perspect Biol.
2011; 3
(6): pii: a005744. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Krug M, Lössner B, Ott T:
Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats.
Brain Res Bull.
1984; 13: 39–42. PubMed Abstract
| Publisher Full Text
- 10.
Matthies H:
In search of cellular mechanisms of memory.
Prog Neurobiol.
1989; 32: 277–349. PubMed Abstract
| Publisher Full Text
- 11.
Frey U, Frey S, Schollmeier F, et al.:
Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro.
J Physiol.
1996; 490
(Pt 3): 703–11. PubMed Abstract
| Free Full Text
- 12.
Nguyen PV, Abel T, Kandel ER:
Requirement of a critical period of transcription for induction of a late phase of LTP.
Science.
1994; 265: 1104–1107. PubMed Abstract
| Publisher Full Text
- 13.
Alberini CM:
Transcription factors in long-term memory and synaptic plasticity.
Physiol Rev.
2009; 89: 121–45. PubMed Abstract
| Publisher Full Text
- 14.
Greenberg ME, Ziff EB, Greene LA:
Stimulation of neuronal acetylcholine receptors induces rapid gene transcription.
Science.
1986; 234: 80–3. PubMed Abstract
| Publisher Full Text
- 15.
Miranti CK, Ginty DD, Huang G, et al.:
Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca2+/calmodulin-dependent kinase.
Mol Cell Biol.
1995; 15: 3672–84. PubMed Abstract
| Free Full Text
- 16.
Guzowski JF, McNaughton BL, Barnes CA, et al.:
Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles.
Nat Neurosci.
1999; 2: 1120–4. PubMed Abstract
| Publisher Full Text
- 17.
Jones MW, Errington ML, French PJ, et al.:
A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories.
Nat Neurosci.
2001; 4: 289–96. PubMed Abstract
| Publisher Full Text
- 18.
Jordan BA, Kreutz MR:
Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling.
Trends Neurosci.
2009; 32: 392–401. PubMed Abstract
| Publisher Full Text
- 19.
Hagenston AM, Bading H:
Calcium signaling in synapse-to-nucleus communication.
Cold Spring Harb Perspect Biol.
2011; 3: a004564. PubMed Abstract
| Publisher Full Text
- 20.
Ch’ng TH, Martin KC:
Synapse-to-nucleus signaling.
Curr Opin Neurobiol.
2011; 21: 345–52. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Pawson T, Scott JD:
Signaling Through Scaffold, Anchoring, and Adaptor Proteins.
Science.
1997; 278: 2075–2080. PubMed Abstract
| Publisher Full Text
- 22.
Ferrell JE Jr:
How regulated protein translocation can produce switch-like responses.
Trends Biochem Sci.
1998; 23: 461–5. PubMed Abstract
| Publisher Full Text
- 23.
Smith FD, Scott JD:
Signaling Complexes: Junctions on the Intracellular Information Super Highway.
Curr Biol.
2002; 12: R32–R40. PubMed Abstract
| Publisher Full Text
- 24.
Teruel MN, Meyer T:
Translocation and reversible localization of signaling proteins: a dynamic future for signal transduction.
Cell.
2000; 103: 181–4. PubMed Abstract
| Publisher Full Text
- 25.
Hagiwara M, Brindle P, Harootunian A, et al.:
Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A.
Mol Cell Biol.
1993; 13: 4852–9. PubMed Abstract
| Free Full Text
- 26.
Davis RJ:
Transcriptional regulation by MAP kinases.
Mol Reprod Dev.
1995; 42: 459–67. PubMed Abstract
| Publisher Full Text
- 27.
Gudi T, Lohmann SM, Pilz RB:
Regulation of gene expression by cyclic GMP-dependent protein kinase requires nuclear translocation of the kinase: identification of a nuclear localization signal.
Mol Cell Biol.
1997; 17: 5244–54. PubMed Abstract
| Free Full Text
- 28.
Meinkoth JL, Ji Y, Taylor SS, et al.:
Dynamics of the distribution of cyclic AMP-dependent protein kinase in living cells.
Proc Natl Acad Sci U S A.
1990; 87: 9595–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Chen RH, Sarnecki C, Blenis J:
Nuclear localization and regulation of erk- and rsk-encoded protein kinases.
Mol Cell Biol.
1992; 12: 915–27. PubMed Abstract
| Free Full Text
- 30.
Shirakawa F, Mizel SB:
In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C.
Mol Cell Biol.
1989; 9: 2424–30. PubMed Abstract
| Free Full Text
- 31.
Miller JR, Moon RT:
Analysis of the signaling activities of localization mutants of beta-catenin during axis specification in Xenopus.
J Cell Biol.
1997; 139: 229–43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 32.
Rao A, Luo C, Hogan PG:
Transcription factors of the NFAT family: regulation and function.
Annu Rev Immunol.
1997; 15: 707–47. PubMed Abstract
| Publisher Full Text
- 33.
Hubbard MJ, Cohen P:
On target with a new mechanism for the regulation of protein phosphorylation.
Trends Biochem Sci.
1993; 18: 172–7. PubMed Abstract
| Publisher Full Text
- 34.
Pawson T, Scott JD:
Signaling through scaffold, anchoring, and adaptor proteins.
Science.
1997; 278: 2075–80. PubMed Abstract
| Publisher Full Text
- 35.
Martin KC, Michael D, Rose JC, et al.:
MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia.
Neuron.
1997; 18: 899–912. PubMed Abstract
| Publisher Full Text
- 36.
Deisseroth K, Heist EK, Tsien RW:
Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons.
Nature.
1998; 392: 198–202. PubMed Abstract
| Publisher Full Text
- 37.
Valjent E, Corvol JC, Pagès C, et al.:
Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties.
J Neurosci.
2000; 20: 8701–9. PubMed Abstract
- 38.
Valjent E, Pagès C, Hervé D, et al.:
Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain.
Eur J Neurosci.
2004; 19: 1826–36. PubMed Abstract
| Publisher Full Text
- 39.
Bertran-Gonzalez J, Bosch C, Maroteaux M, et al.:
Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol.
J Neurosci.
2008; 28: 5671–85. PubMed Abstract
| Publisher Full Text
- 40.
Shiflett MW, Brown RA, Balleine BW:
Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats.
J Neurosci.
2010; 30: 2951–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 41.
Matamales M, Girault JA:
Signaling from the cytoplasm to the nucleus in striatal medium-sized spiny neurons.
Front Neuroanat.
2011; 5: 37. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Suzuki T:
Messengers from the synapse to the nucleus (MSNs) that establish late phase of long-term potentiation (LTP) and long-term memory.
Neurosci Res.
1996; 25: 1–6. PubMed Abstract
| Publisher Full Text
- 43.
Meberg PJ, Kinney WR, Valcourt EG, et al.:
Gene expression of the transcription factor NF-kappa B in hippocampus: regulation by synaptic activity.
Brain Res Mol Brain Res.
1996; 38: 179–90. PubMed Abstract
| Publisher Full Text
- 44.
Hirokawa N:
Kinesin and Dynein Superfamily Proteins and the Mechanism of Organelle Transport.
Science.
1998; 279: 519–526. PubMed Abstract
| Publisher Full Text
- 45.
Zweifel LS, Kuruvilla R, Ginty DD:
Functions and mechanisms of retrograde neurotrophin signalling.
Nat Rev Neurosci.
2005; 6: 615–25. PubMed Abstract
| Publisher Full Text
- 46.
Deisseroth K, Mermelstein PG, Xia H, et al.:
Signaling from synapse to nucleus: the logic behind the mechanisms.
Curr Opin Neurobiol.
2003; 13: 354–365. PubMed Abstract
| Publisher Full Text
- 47.
Kaltschmidt C, Kaltschmidt B, Baeuerle PA:
Brain synapses contain inducible forms of the transcription factor NF-κB.
Mech Dev.
1993; 43: 135–147. PubMed Abstract
| Publisher Full Text
- 48.
Suzuki T, Mitake S, Okumura-Noji K, et al.:
Presence of NF-κB-like and IκB-like immunoreactivities in postsynaptic densities.
NeuroReport.
1997; 8: 2931–2935. PubMed Abstract
- 49.
Suzuki T, Usuda N, Ishiguro H, et al.:
Occurrence of a transcription factor, cAMP response element-binding protein (CREB), in the postsynaptic sites of the brain.
Brain Res Mol Brain Res.
1998; 61: 69–77. PubMed Abstract
| Publisher Full Text
- 50.
Lai KO, Zhao Y, Ch’ng TH, et al.:
Importin-mediated retrograde transport of CREB2 from distal processes to the nucleus in neurons.
Proc Natl Acad Sci U S A.
2008; 105: 17175–80. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 51.
Ch’ng TH, Uzgil B, Lin P, et al.:
Activity-Dependent Transport of the Transcriptional Coactivator CRTC1 from Synapse to Nucleus.
Cell.
2012; 150: 207–21. PubMed Abstract
| Publisher Full Text
- 52.
Thompson KR, Otis KO, Chen DY, et al.:
Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway.
Neuron.
2004; 44: 997–1009. PubMed Abstract
| Publisher Full Text
- 53.
Otis KO, Thompson KR, Martin KC:
Importin-mediated nuclear transport in neurons.
Curr Opin Neurobiol.
2006; 16: 329–335. PubMed Abstract
| Publisher Full Text
- 54.
Ström AC, Weis K:
Importin-beta-like nuclear transport receptors.
Genome Biol.
2001; 2: REVIEWS3008. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 55.
Mosammaparast N, Pemberton LF:
Karyopherins: from nuclear-transport mediators to nuclear-function regulators.
Trends Cell Biol.
2004; 14: 547–56. PubMed Abstract
| Publisher Full Text
- 56.
Kalderon D, Roberts BL, Richardson WD, et al.:
A short amino acid sequence able to specify nuclear location.
Cell.
1984; 39: 499–509. PubMed Abstract
| Publisher Full Text
- 57.
Robbins J, Dilworth SM, Laskey RA, et al.:
Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence.
Cell.
1991; 64: 615–23. PubMed Abstract
| Publisher Full Text
- 58.
Marsh DR, Holmes KD, Dekaban GA, et al.:
Distribution of an NMDA receptor:GFP fusion protein in sensory neurons is altered by a C-terminal construct.
J Neurochem.
2001; 77: 23–33. PubMed Abstract
| Publisher Full Text
- 59.
Holmes KD, Mattar P, Marsh DR, et al.:
The C-terminal C1 cassette of the N -methyl-D-aspartate receptor 1 subunit contains a bi-partite nuclear localization sequence.
J Neurochem.
2002; 81: 1152–65. PubMed Abstract
| Publisher Full Text
- 60.
Jeffrey RA, Ch’ng TH, O’Dell TJ, et al.:
Activity-dependent anchoring of importin alpha at the synapse involves regulated binding to the cytoplasmic tail of the NR1-1a subunit of the NMDA receptor.
J Neurosci.
2009; 29: 15613–20. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 61.
Jordan BA, Ziff EB:
To the Nucleus with Proteomics.
Transcriptional Reg Neuronal Activity.
2008; 27–50. Publisher Full Text
- 62.
Jordan BA, Fernholz BD, Boussac M, et al.:
Identification and verification of novel rodent postsynaptic density proteins.
Mol Cell Proteomics.
2004; 3: 857–71. PubMed Abstract
| Publisher Full Text
- 63.
Jordan BA, Fernholz BD, Khatri L, et al.:
Activity-dependent AIDA-1 nuclear signaling regulates nucleolar numbers and protein synthesis in neurons.
Nat Neurosci.
2007; 10: 427–35. PubMed Abstract
| Publisher Full Text
- 64.
Jordan BA, Fernholz BD, Neubert TA, et al.:
New tricks for an old dog: proteomics of the PSD.The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology, 2006. Reference Source
- 65.
Ubersax JA, Ferrell JE Jr:
Mechanisms of specificity in protein phosphorylation.
Nat Rev Mol Cell Biol.
2007; 8: 530–41. PubMed Abstract
| Publisher Full Text
- 66.
Kennelly PJ, Krebs EG:
Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases.
J Biol Chem.
1991; 266: 15555–8. PubMed Abstract
- 67.
Moore MS, Schwoebel ED:
Nuclear import in digitonin-permeabilized cells.
Curr Protoc Cell Biol.
2001; Chapter 11, Unit 11.7. PubMed Abstract
| Publisher Full Text
- 68.
Marshall KS, Zhang Z, Curran J, et al.:
An improved genetic system for detection and analysis of protein nuclear import signals.
BMC Mol Biol.
2007; 8: 6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 69.
Schnell U, Dijk F, Sjollema KA, et al.:
Immunolabeling artifacts and the need for live-cell imaging.
Nat Methods.
2012; 9: 152–8. PubMed Abstract
| Publisher Full Text
- 70.
Huang B, Babcock H, Zhuang X:
Breaking the diffraction barrier: super-resolution imaging of cells.
Cell.
2010; 143: 1047–58. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 71.
Adams JP, Dudek SM:
Late-phase long-term potentiation: getting to the nucleus.
Nat Rev Neurosci.
2005; 6: 737–43. PubMed Abstract
| Publisher Full Text
- 72.
Adams JP, Robinson RA, Dudek SM:
Role of Action Potentials in Regulating Gene Transcription: Relevance to LTP.
Transcriptional Reg Neuronal Activity.
2008; 91–110. Publisher Full Text
- 73.
Hardingham GE, Arnold FJ, Bading H:
Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity.
Nat Neurosci.
2001; 4: 261–7. PubMed Abstract
| Publisher Full Text
- 74.
Greer PL, Greenberg ME:
From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function.
Neuron.
2008; 59: 846–60. PubMed Abstract
| Publisher Full Text
- 75.
Zhang SJ, Zou M, Lu L, et al.:
Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity.
PLoS Genet.
2009; 5: e1000604. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 76.
Berridge MJ:
Neuronal calcium signaling.
Neuron.
1998; 21: 13–26. PubMed Abstract
| Publisher Full Text
- 77.
Petersen OH, Tepikin A, Park MK:
The endoplasmic reticulum: one continuous or several separate Ca(2+) stores?
Trends Neurosci.
2001; 24: 271–6. PubMed Abstract
| Publisher Full Text
- 78.
Martone ME, Zhang Y, Simpliciano VM, et al.:
Three-dimensional visualization of the smooth endoplasmic reticulum in Purkinje cell dendrites.
J Neurosci.
1993; 13: 4636–46. PubMed Abstract
- 79.
Terasaki M, Slater NT, Fein A, et al.:
Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons.
Proc Natl Acad Sci U S A.
1994; 91: 7510–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 80.
Streb H, Irvine RF, Berridge MJ, et al.:
Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate.
Nature.
1983; 306: 67–9. PubMed Abstract
| Publisher Full Text
- 81.
Abe T, Sugihara H, Nawa H, et al.:
Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction.
J Biol Chem.
1992; 267: 13361–8. PubMed Abstract
- 82.
Pin JP, Joly C, Heinemann SF, et al.:
Domains involved in the specificity of G protein activation in phospholipase C-coupled metabotropic glutamate receptors.
EMBO J.
1994; 13: 342–8. PubMed Abstract
| Free Full Text
- 83.
Finch EA, Turner TJ, Goldin SM:
Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release.
Science.
1991; 252: 443–6. PubMed Abstract
| Publisher Full Text
- 84.
Endo M, Tanaka M, Ogawa Y:
Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres.
Nature.
1970; 228: 34–6. Publisher Full Text
- 85.
Ford LE, Podolsky RJ:
Regenerative calcium release within muscle cells.
Science.
1970; 167: 58–9. PubMed Abstract
| Publisher Full Text
- 86.
Endo M:
Calcium-induced calcium release in skeletal muscle.
Physiol Rev.
2009; 89: 1153–76. PubMed Abstract
| Publisher Full Text
- 87.
Bloodgood BL, Sabatini BL:
Ca(2+) signaling in dendritic spines.
Curr Opin Neurobiol.
2007; 17: 345–51. PubMed Abstract
| Publisher Full Text
- 88.
Kerr JN, Denk W:
Imaging in vivo: watching the brain in action.
Nat Rev Neurosci.
2008; 9: 195–205. PubMed Abstract
| Publisher Full Text
- 89.
Helmchen F:
Two-photon functional imaging of neuronal activity. In Vivo Optical Imaging of Brain Function2009. Reference Source
- 90.
Ross WN, Manita S:
Imaging calcium waves and sparks in central neurons.
Cold Spring Harb Protoc.
2012; 2012: 1087–91. PubMed Abstract
| Publisher Full Text
- 91.
Miller LD, Petrozzino JJ, Golarai G, et al.:
Ca2+ release from intracellular stores induced by afferent stimulation of CA3 pyramidal neurons in hippocampal slices.
J Neurophysiol.
1996; 76: 554–62. PubMed Abstract
- 92.
Larkum ME, Watanabe S, Nakamura T, et al.:
Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons.
J Physiol.
2003; 549: 471–88. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 93.
Nakamura T, Barbara JG, Nakamura K, et al.:
Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials.
Neuron.
1999; 24: 727–37. PubMed Abstract
| Publisher Full Text
- 94.
Hagenston AM, Fitzpatrick JS, Yeckel MF:
MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons.
Cereb Cortex.
2008; 18: 407–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 95.
Power JM, Sah P:
Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons.
J Neurosci.
2008; 28: 3209–20. PubMed Abstract
| Publisher Full Text
- 96.
Ross WN:
Understanding calcium waves and sparks in central neurons.
Nat Rev Neurosci.
2012; 13: 157–68. PubMed Abstract
| Publisher Full Text
- 97.
Fitzpatrick JS, Hagenston AM, Hertle DN, et al.:
Inositol-1,4,5-trisphosphate receptor-mediated Ca2+ waves in pyramidal neuron dendrites propagate through hot spots and cold spots.
J Physiol.
2009; 587: 1439–59. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 98.
Jaffe DB, Brown TH:
Metabotropic glutamate receptor activation induces calcium waves within hippocampal dendrites.
J Neurophysiol.
1994; 72: 471–4. PubMed Abstract
- 99.
Power JM, Sah P:
Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons.
J Neurosci.
2002; 22: 3454–62. PubMed Abstract
- 100.
Nakamura T, Nakamura K, Lasser-Ross N, et al.:
Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons.
J Neurosci.
2000; 20: 8365–76. PubMed Abstract
- 101.
Ross CA, Meldolesi J, Milner TA, et al.:
Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons.
Nature.
1989; 339: 468–70. PubMed Abstract
| Publisher Full Text
- 102.
Satoh T, Ross CA, Villa A, et al.:
The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment.
J Cell Biol.
1990; 111: 615–24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 103.
Marchenko SM, Thomas RC:
Nuclear Ca2+ signalling in cerebellar Purkinje neurons.
Cerebellum.
2006; 5: 36–42. PubMed Abstract
| Publisher Full Text
- 104.
Imagawa T, Smith JS, Coronado R, et al.:
Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel.
J Biol Chem.
1987; 262: 16636–43. PubMed Abstract
- 105.
Hymel L, Inui M, Fleischer S, et al.:
Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers.
Proc Natl Acad Sci U S A.
1988; 85: 441–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 106.
Lai FA, Erickson HP, Rousseau E, et al.:
Purification and reconstitution of the calcium release channel from skeletal muscle.
Nature.
1988; 331: 315–9. PubMed Abstract
| Publisher Full Text
- 107.
Bezprozvanny I, Watras J, Ehrlich BE:
Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum.
Nature.
1991; 351: 751–4. PubMed Abstract
| Publisher Full Text
- 108.
Finch EA, Turner TJ, Goldin SM:
Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release.
Science.
1991; 252: 443–6. PubMed Abstract
| Publisher Full Text
- 109.
Iino M:
Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci.
J Gen Physiol.
1990; 95: 1103–22. PubMed Abstract
| Free Full Text
- 110.
Iino M:
Molecular basis of spatio-temporal dynamics in inositol 1,4,5-trisphosphate-mediated Ca2+ signalling.
Jpn J Pharmacol.
2000; 82: 15–20. PubMed Abstract
- 111.
Foskett JK, White C, Cheung KH, et al.:
Inositol trisphosphate receptor Ca2+ release channels.
Physiol Rev.
2007; 87: 593–658. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 112.
Allbritton NL, Meyer T, Stryer L:
Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate.
Science.
1992; 258: 1812–5. PubMed Abstract
| Publisher Full Text
- 113.
Wang SS, Alousi AA, Thompson SH:
The lifetime of inositol 1,4,5-trisphosphate in single cells.
J Gen Physiol.
1995; 105: 149–71. PubMed Abstract
| Free Full Text
- 114.
Helmchen F:
Biochemical compartmentalization in dendrites. Dendrites. 2007. Publisher Full Text
- 115.
O’Malley KL, Jong YJ, Gonchar Y, et al.:
Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons.
J Biol Chem.
2003; 278: 28210–9. PubMed Abstract
| Publisher Full Text
- 116.
Jong YJ, Kumar V, Kingston AE, et al.:
Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand.
J Biol Chem.
2005; 280: 30469–80. PubMed Abstract
| Publisher Full Text
- 117.
Jong YJ, Schwetye KE, O’Malley KL:
Nuclear localization of functional metabotropic glutamate receptor mGlu1 in HEK293 cells and cortical neurons: role in nuclear calcium mobilization and development.
J Neurochem.
2007; 101: 458–69. PubMed Abstract
| Publisher Full Text
- 118.
Marchenko SM, Yarotskyy VV, Kovalenko TN, et al.:
Spontaneously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurones.
J Physiol.
2005; 565: 897–910. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 119.
Dudek SM, Fields RD:
Somatic action potentials are sufficient for late-phase LTP-related cell signaling.
Proc Natl Acad Sci U S A.
2002; 99: 3962–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 120.
Adams JP, Robinson RA, Hudgins ED, et al.:
NMDA receptor-independent control of transcription factors and gene expression.
Neuroreport.
2009; 20: 1429–1433. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 121.
Eshete F, Fields RD:
Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons.
J Neurosci.
2001; 21: 6694–705. PubMed Abstract
- 122.
Zhao M, Adams JP, Dudek SM:
Pattern-dependent role of NMDA receptors in action potential generation: consequences on extracellular signal-regulated kinase activation.
J Neurosci.
2005; 25: 7032–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 123.
Saha RN, Dudek SM:
Action potentials: to the nucleus and beyond.
Exp Biol Med (Maywood).
2008; 233: 385–93. PubMed Abstract
| Publisher Full Text
- 124.
Hausser M, Spruston N, Stuart GJ:
Diversity and Dynamics of Dendritic Signaling.
Science.
2000; 290: 739–744. PubMed Abstract
| Publisher Full Text
- 125.
Spruston N, Stuart GJ, Häusser M:
Dendritic integration. Dendrites. 2007. Publisher Full Text
- 126.
Golding NL, Staff NP, Spruston N:
Dendritic spikes as a mechanism for cooperative long-term potentiation.
Nature.
2002; 418: 326–31. PubMed Abstract
| Publisher Full Text
- 127.
Kampa BM, Letzkus JJ, Stuart GJ:
Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity.
J Physiol.
2006; 574: 283–90. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 128.
Stuart GJ, Häusser M:
Dendritic coincidence detection of EPSPs and action potentials.
Nat Neurosci.
2001; 4: 63–71. PubMed Abstract
| Publisher Full Text
- 129.
Segev I, Rall W:
Excitable dendrites and spines: earlier theoretical insights elucidate recent direct observations.
Trends Neurosci.
1998; 21: 453–60. PubMed Abstract
| Publisher Full Text
- 130.
Vetter P, Roth A, Häusser M:
Propagation of action potentials in dendrites depends on dendritic morphology.
J Neurophysiol.
2001; 85: 926–37. PubMed Abstract
- 131.
Westenbroek RE, Ahlijanian MK, Catterall WA:
Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons.
Nature.
1990; 347: 281–4. PubMed Abstract
| Publisher Full Text
- 132.
Murphy TH, Worley PF, Baraban JM:
L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes.
Neuron.
1991; 7: 625–35. PubMed Abstract
| Publisher Full Text
- 133.
Dolmetsch RE, Pajvani U, Fife K, et al.:
Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway.
Science.
2001; 294: 333–9. PubMed Abstract
| Publisher Full Text
- 134.
Gallin WJ, Greenberg ME:
Calcium regulation of gene expression in neurons: the mode of entry matters.
Curr Opin Neurobiol.
1995; 5: 367–74. PubMed Abstract
| Publisher Full Text
- 135.
Wheeler DG, Groth RD, Ma H, et al.:
Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression.
Cell.
2012; 149: 1112–24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 136.
Wiegert JS, Bading H:
Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation.
Cell Calcium.
2011; 49: 296–305. PubMed Abstract
| Publisher Full Text



Comments on this article Comments (0)