Keywords
Aminooctylylphosphonic acid, solvent extraction, ion-exchange mechanism, Pb (II), Cd (II)
Aminooctylylphosphonic acid, solvent extraction, ion-exchange mechanism, Pb (II), Cd (II)
The most rejected heavy metals are very polluting and dangerous. Lead and its inorganic derivatives are classified as category 2B (potentially carcinogenic to humans).1 Lead modifies the cellular functioning by disrupting different physiological processes. It can thus cause blood anemia and renal effects (renal failure). Exposure to lead can affect the central nervous system (developmental delay, irritability, sleep disorders, loss of memory) and have long-term effects on fertility.2 The effects of lead are generally amplified in the fetus and child (congenital abnormalities, persistent neurobehavioral deficits).2,3 Cadmium, used in several industrial and agricultural processes as well as mining activities and designed carcinogenic by the United States (US) National Toxicology Program can increase the contamination of water and wastewaters around the world.4,5 Cadmium can produce or increase the incidence of non-hereditary adverse effects in offspring.6 Inhalation, ingestion or skin penetration, even in very small quantities, can cause irritation in the stomach, leading to vomiting and diarrhea, renal insufficiency and, at high doses, chronic bronchitis, fibrosis, emphysema, damage to the bone system, kidney stones and increased blood pressure.7–10
In order to eliminate or at least reduce the effects of these cations, many processes have been developed to face dangers towards human health and environment.
The use of the extraction process was widely used thus occupying a special place (for analytical purposes, in the processing of nuclear raw materials and in industries related to the fuel cycle). Solvent extraction consists of treating aqueous industrial waste before it is released into the environment. This process is a very efficient way of upgrading or rendering industrial wastes inert, and to treat discharges contaminated with lead and cadmium, and is generally aimed at recovering these metals, in order to reuse them and thus reduce their eco-toxicological impact.11-13
The aim of our work was to determine the optimal parameters, at which the extraction yield was maximum, of the solvent extraction of lead (II) and cadmium (II) from an aqueous solution in nitrate medium by a new chelating agent- aminooctyldiphosphonic acid (AAODMDP), dissolved in chloroform, synthesized under microwaves.14
This study was conducted in April 2020 in our laboratory and LCMT Caen (France). The experimental procedure and metal cations analysis is detailed below. In brief after preparing aqueous and organic solutions, contact between the solutions was done. The two phases were separated gravimetrically, and 1 mL of aqueous solution was taken for UV/V analysis.
For synergetic effect, the organic phase was a mixture of our chelating agent (AODMDPA) and a solvating agent (tri octyl phosphine oxide) at different volume ratio.
The reagents used in this study (with supplier and catalogue number) were: Lead nitrate tetrahydrate Pb (NO3)2 4H2O (99%, Riedel De Haen, 10099-74-8), Cadmium nitrate tetrahydrate Cd (NO3)2 4H2O (99%, Riedel De Haen, 10022-68-1), Arsenazo III C22H18AS2N4O14S2 (99%, Aldrich, 216-788-6), Nitric acid (65%, Panreac, 7697 37 2), Acétic acid CH3COOH (80%, prolabo, 64-19-7), Sodium Acétate CH3COONa (98%, prolabo, 6131-90-4), Potassium nitrate KNO3 (99%, Riedel De Haen, 7757-79-1),Chloroform CHCl3 (99% Riedel De Haen, 67-66-3), Aminooctane C8H19N (Aldrich, 111-86-4), Phosphorus acid (H3PO3, Aldrich, 13598-36-2), Hydrochloric acid (HCl, Riedel de Haen, 7647-01-0) , Formaldehyde (HCHO, Aldrich, 50-00-0), Acetone (C3H6O, Aldrich, 67-64-1), Tri octyl phosphine oxide TOPO ((C8H17)3PO, 78-50-2) and aminooctyldiphosphonic acid (AODPA).14 Microwave irradiations were performed using microwave oven Synthewave 402 (Prolabo) working at a frequency of 2450 MHz. Nuclear magnetic resonance (NMR) spectra were done on a Fourier Bruker AC multinuclear spectrometer. Ultraviolet–visible spectrophotometry (UV/Vis) spectra were obtained using the UV/Visdouble beam Optizen 3220UVspectrometer, a digital pH meter type Consort C863 to follow solution pH.
A mixture of aminooctane (C4H11N) (4.96 mL, 30.0 mmol), phosphorus acid (H3PO3) (5.02 g, 60.0 mmol), water (3.0 mL) and hydrochloric acid (HCL) 37% (3.0 mL) was irradiated (in a microwave oven) in a glass cylinder reactor fitted with a cooler at 240 W for 2 min. After adding formaldehyde (HCHO) 37% (4.8 mL, 64.46 mmol) rapidly, the mixture was irradiated for 12 min at 240 W. After cooling and evaporation for 5 min, the precipitate was filtered and the white solid was washed with acetone and water.
The product had the following properties: Yield (92 %), mp > 240°C, Formula: C10H25NO6P2, 1H NMR (D2O, Na2CO3):1.07 (t, 3H, CH3), 1.15 (m, 12H, CH2), 3.2 (d, 2JHP = 9,01 NCH2-P), 3.46 (m, 2H, N-CH2); 31P NMR (D2O, Na2CO3): d/H3PO4 (ppm) s, 6.7; 13CNMR (D2O, Na2CO3): 19 (s, C1), 25.4 (s, C2), 27.3(s, C3), 31.7 (s, C3), 51 (d, 2JCP = 137.7, NCH2-P); IR (υ Cm−1): 2925(νasCH), 1333 (Deformation of (CH2)n: n > 4) 1120 (νs P-OH), 1044 (νs P=O), 938 (νsCm υ P-O) (s:symetric, as: antisymetric); pKa: 2.75, 8.73, 9.35, (9.67). These values indicated that in the water–acetone media, the first proton was strong and the other protons were weak.
After dissolving aminooctyldiphosphonic acid in chloroform, the obtained organic solutions were used for extraction studies. Aqueous metal solutions were prepared by dissolving Pb (NO3)2 4H2O and Cd (NO3)2 4H2O in distilled water. The first stage consisted of achieving curves of standardization of the absorbance according to the concentration of Pb2+ and Cd2+. The concentrations were in the range 10−6-10−3 mol. L−1. Pb2+ and Cd 2+ analyses in the aqueous phase after extraction was performed by the Arsenazo III spectrophotometric method which consists of using this chromogenic reagent to form ion-arsenazo III complex. After separation of the organic from the aqueous phase, Arsenazo III was added to the later to form Arsenazo-Ion complex, which was placed in a quartz cuvette after adjusting the aqueous solution pH with buffer solution. The absorbance was learnt at λmax = 610 nm for Pb2+ (pH = 4)15 and λmax = 600 nm for Cd2+ (pH = 9.5),16 the second stage involved optimizing factors of extraction. The volume ratio Vaq/Vorg (the two phases: aqueous and organic; were mixed with different volumes) was carried out to establish the optimal yield of extraction and avoid emulsions, followed by the extraction kinetics (the two phases were stirred for different time). This was carried out to determine the necessary optimal time to reach the extraction equilibrium and for which the yield is maximal. The effect of different concentrations of the extractant (10−3M - 10−6M) on the extraction of the metallic ions was studied. The effect of the ionic strength (a salt (KNO3) was added to the aqueous phase) and medium acidity (adding an acid (HNO3) to the aqueous phase at a specific pH) were investigated adding salt or acids to aqueous solutions respectively. Synergetic effect was investigated by adding a solvating agent (Tri octyl phosphine oxide) to the chelating agent (AODPA) in the organic phase then determine the synergetic coefficient.
In order to easily determine the different extraction factors, emulsions must be avoided. The distribution coefficient, D, and the extraction yield, Y, defined as follows,10 will be used to discuss the extraction results:
With the variables being as follows: Abi, Abf are the initial and final absorbance of aqueous solutions before and after extraction respectively.
A parameter that appeared essential is the effect of the stirring speed. We noted that a medium stirring speed gave the highest extraction yield for both cations (Figure 1 and 2).27 This can be explained by the fact that at the lowest speed, and taking into account the ionic radius of Pb2+ or Cd2+, the mass transfer is not favored. Also, at the highest stirring speed, the two cations seemed to be des-extracted which will help us in the des-extraction process.
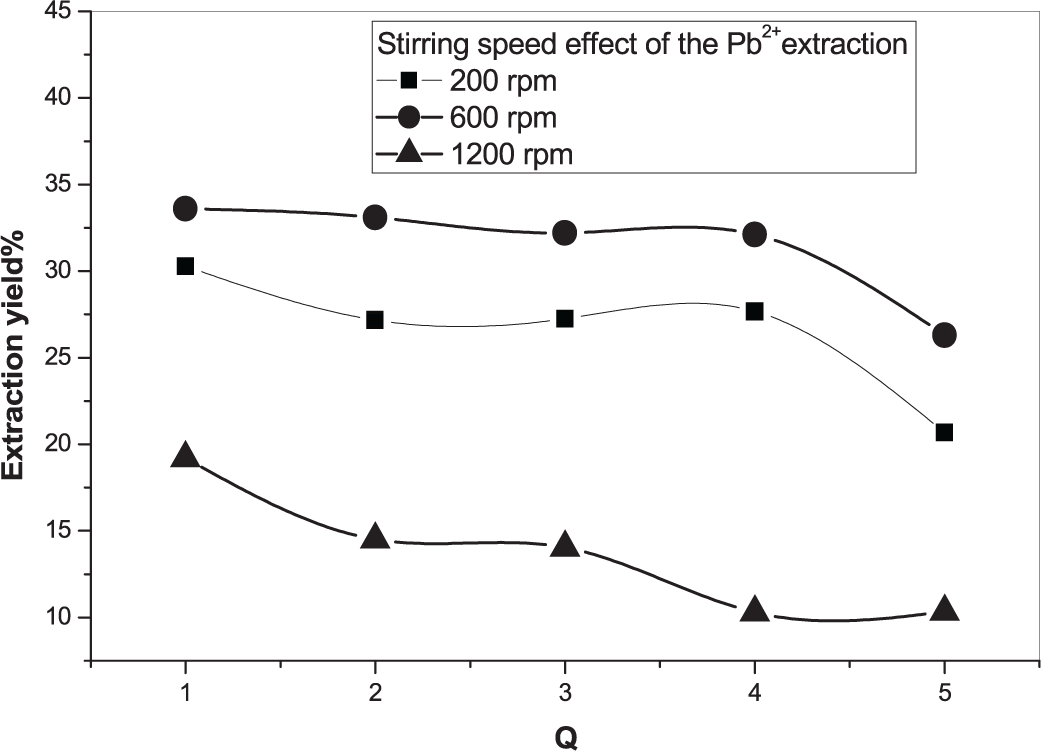
rpm = rotations per minute.
The effect of volume ratio on the extraction yield of Pb2+ or Cd2+ was investigated using lead and cadmium nitrate aqueous solutions of 5×10−4 M. A concentration of 10−3 M of the organic phase was prepared by dissolving aminooctyl diphosphonic acid in chloroform. The results are indicated in Figure 3.
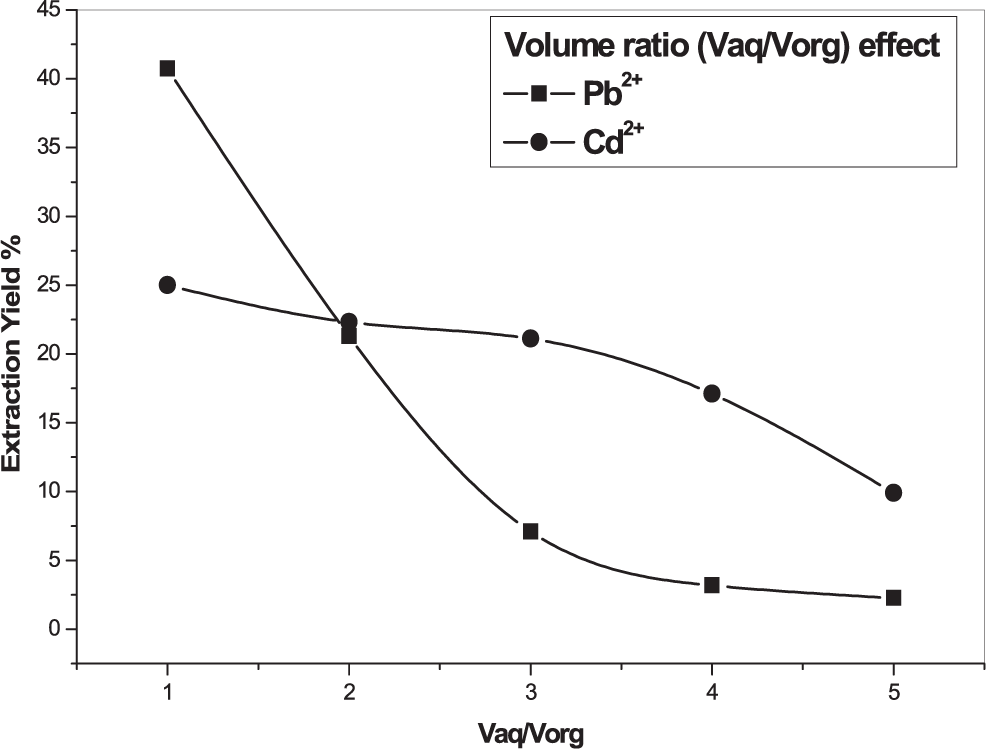
Organic phase [AODMDPA] = M, [Cd2+] = [Pb2+] = M, 600 rpm, T = 25°C.
A volume ratio of 1 (which gave the maximum extraction yield) was used in the following experiences since the best-obtained yield of 40% and 25% were obtained, under this ratio, for Pb (II) and Cd (II) respectively. It is observed from Figure 3 that the higher the volume ratio, the lowest yield was, caused probably by a repulsion effect between the metal ions.
The extraction kinetics results are shown in Figure 4. The variation of the stirring time between 0 and 30 minutes showed that the maximum yield of 32% was obtained for Pb2+ after 15 minutes, and 10 minutes was necessary for the Cd+2. Thus, Pb2+ has greater resistance to mass transfer than Cd2+. This can be explained according to the atomic properties of the ionic radius of lead (1.2 A°) compared to that of cadmium (0.97 A°)
The molar ratio, Q, was used to investigate the extraction yield of the two metal ions. It is expressed as:
It can be seen in Figure 5, that the extraction of the two metal cations decreases with increasing AODMDPA concentration in the organic phase. The best extraction yields obtained were 55 % and 32% for Cd2+ and Pb2+ ions, respectively, at a molar ratio Q = 1.
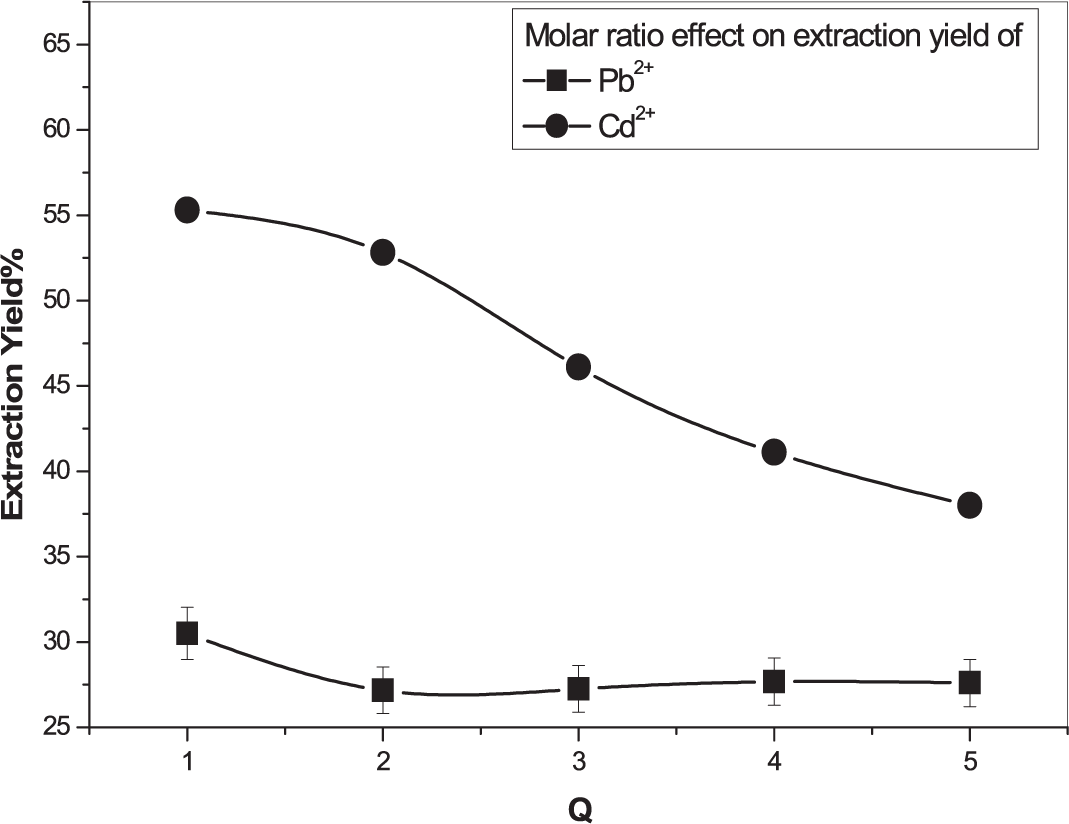
[Pb2+] = [Cd2+] = 5 × 10−4 M, Vaq/Vorg = 1, 600rpm, T = 25°.
Ionic strength measures the electric field tension in a solution. To verify the influence of ionic strength on the output of extraction, we modified the aqueous phase by the addition of KNO3 and HNO3.
Influence of the addition of potassium nitrate (KNO3)
The concentrations of KNO3 were taken equal to 0.01 M, 0.1 M and 1 M. The obtained results are shown in Figures 6 and 7. According to the results, we found that there was an increase in the extraction yield of Pb2+ ions whatever the quantity of KNO3 added to the system. A higher extraction percentage of lead ions in the presence of potassium nitrate can be attributed to the formation of stable complexes of Pb2+ in the aqueous phase in the presence of the latter salt.17 However, Additions of KNO3 reduce the extraction yield in the case of Cd+2. This may be due to the competition between Cd2+ and K+ ions, which is in accordance with the literature.18
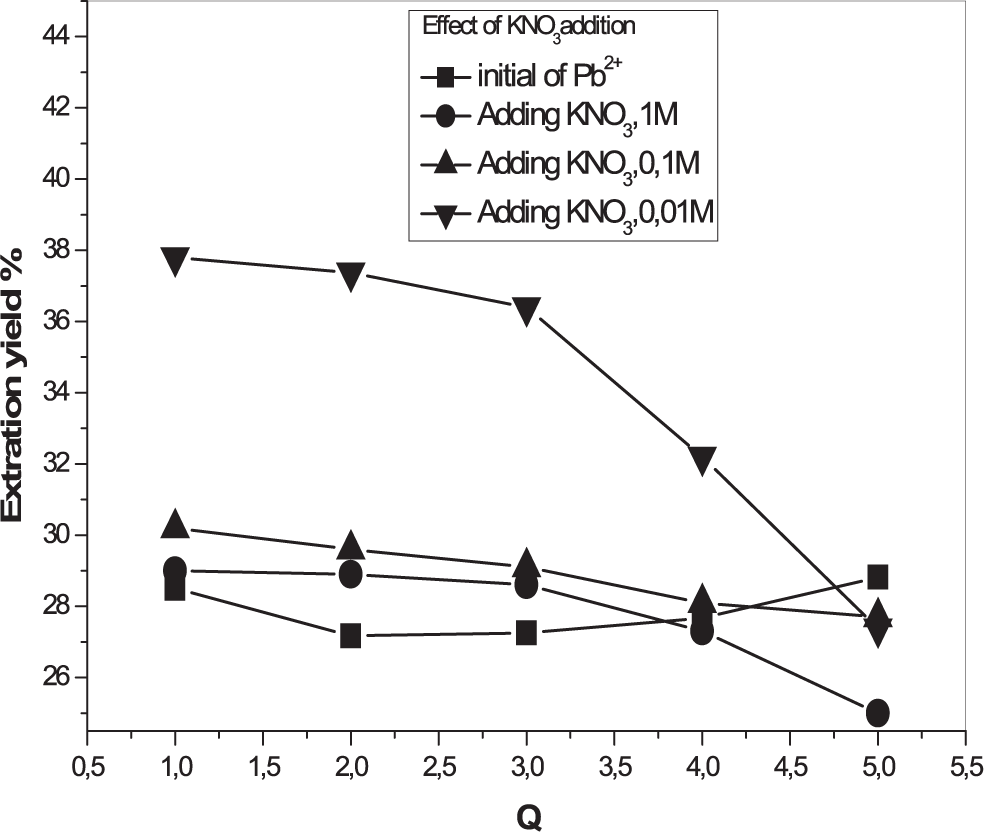
[Pb2+] = 5×10−4 M, Vaq/Vorg = 1, T = 25°C.
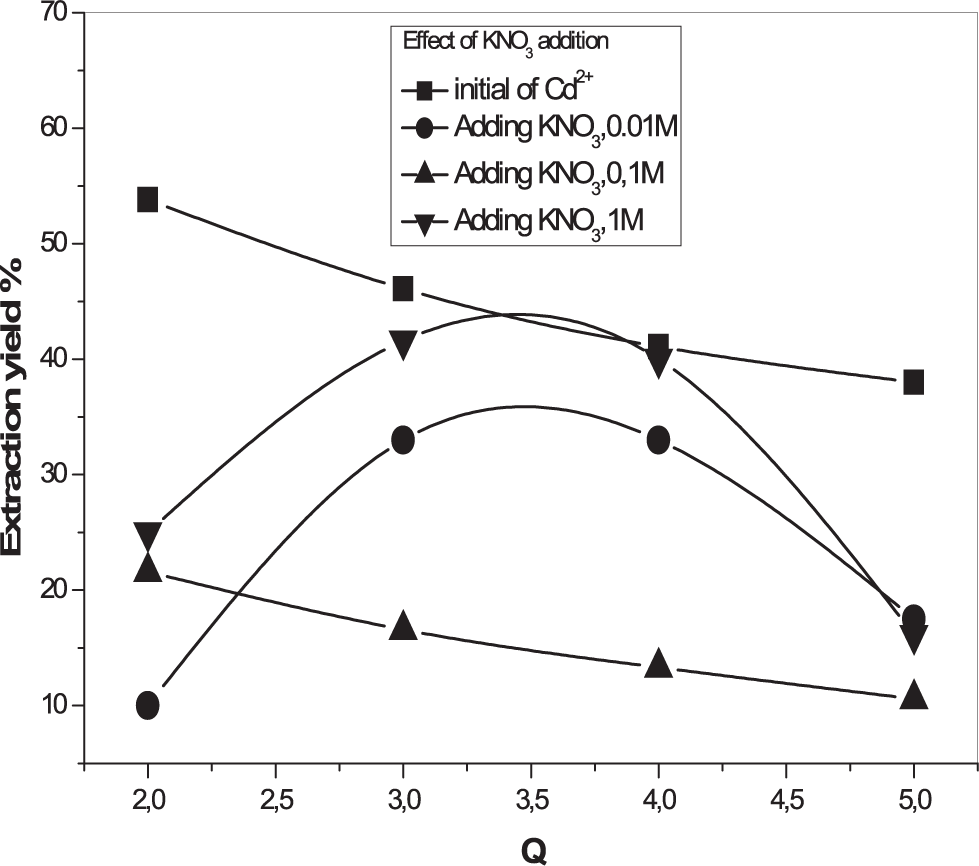
[Cd2+] = 5 × 10−4 M, Vaq/Vorg = 1, T = 25°C.
Influence of the addition of nitric acid (HNO3)
The effect of nitric acid addition to the aqueous phase before extraction was investigated, in the range of pH 2 – 3, on the extraction of 5×10−4 M of metallic cations. The results are shown in Figures 8 and 9. It was noted that the acidic medium disadvantages the lead ions extraction whereas, that of cadmium ions was seen to be favoured. Similar results were obtained in previous papers.19,20
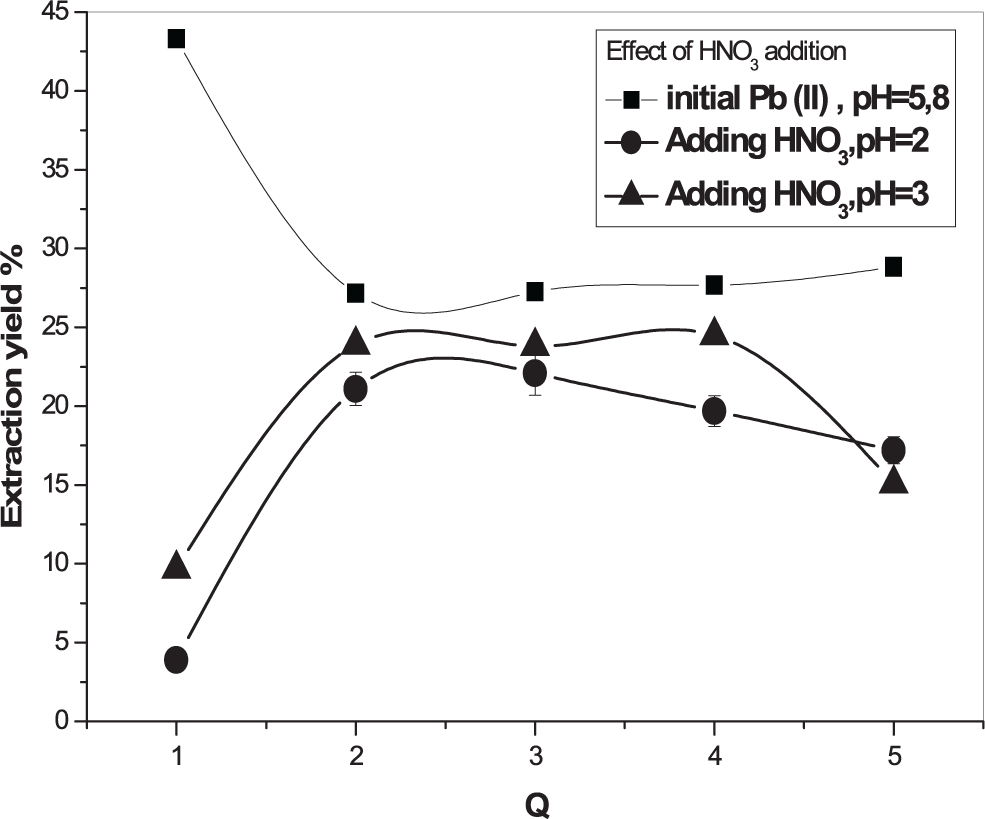
[Pb2+] = 5 × 10−4 M, Vaq/Vorg = 1, T = 25°C.
Another important variable affecting lead and cadmium recovery was studied. In Figures 10, 11, other extraction steps were realized. It was noted that Cd2+ can be recovered completely when Pb2+ reached 73% after two steps.
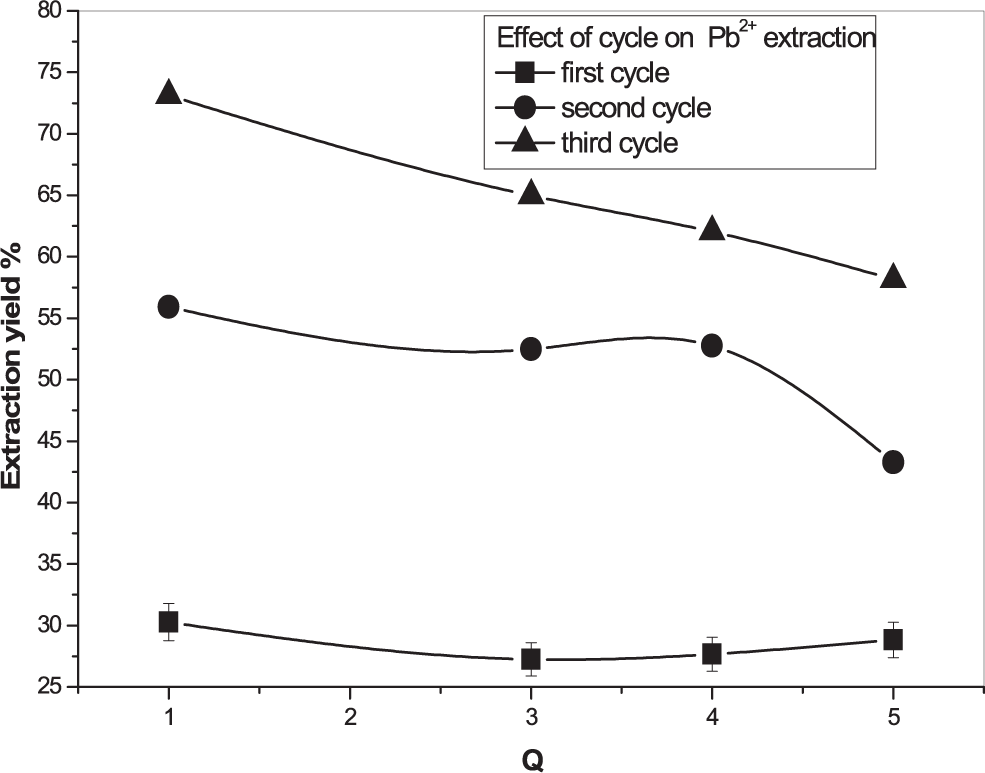
[Pb2+] = 5×10−4 M, Vaq/Vorg = 1, T = 25°C.
The extraction yield was studied in the range of 10 to 40 ± 1°C. The results are given in Figure 12.
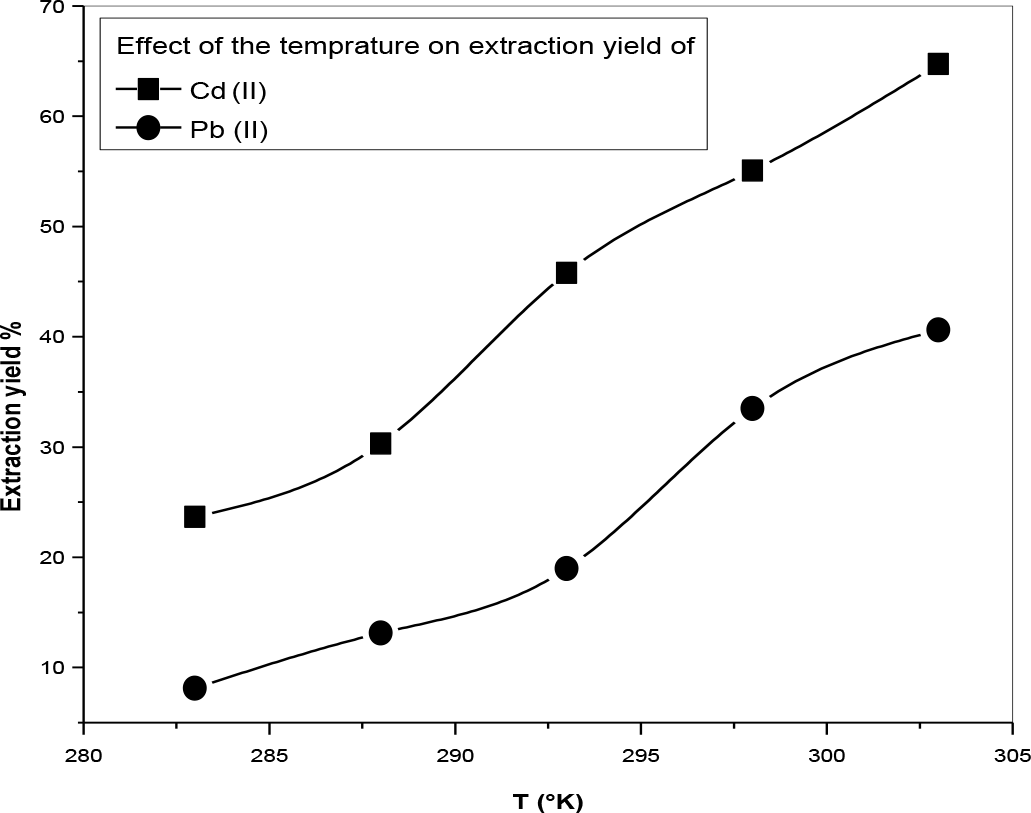
Effect of temperature of the extraction yield of Lead and Cadmium, [AODMDPA] = 10−3M [Pb2+] = [Cd2+] = 5 × 10−4 M, (Vaq/Vorg) = 1.
From Figure 8, it noticed that an increase in the temperature from 10°C to 40°C favoured extraction yield. It allows us to predict the Pb2+ and Cd2+ des-extraction at high temperatures. This may be associated with the increase in the release of water molecules upon dehydration of ions during extraction. This is in good agreement with the results found in the literature.21,22
Plots of the ln K values as a function of the inverse temperature [1/T (K)] in the range 283–313 K gave a straight line (Figure 13). The thermodynamic parameters can be determined by the following expressions23:
From these equations (1) and (2) we pull the following equation (3) to calculate ∆H° and ∆S° while drawing the curve LnK according to the temperature.
The numerical values of ΔH0, ΔS0 are computed from the slope.24 The negative value of Gibbs free energy as shown in Table 1 indicates the spontaneous nature of extraction, while positive value of ΔH0 reflects the endothermic extraction behavior. The positive value of ΔS0 may be due to the increase in randomness around the chelating function.25
In order to study the synergetic effect, Tri octyl phosphine oxide TOPO (solvating agent) was added to our extractant (chelating agent) at 298 K. The synergetic coefficients were obtained as described by M. Taube and al.26
Different phosphonic/TOPO volume ratios were tested presenting negative coefficients maybe because of steric and competition phenomena in the organic phase. The best synergetic coefficient was obtained at a phosphonic acid/TOPO volume ratio of 3 (Figures 14 and 15). The synergistic enhancement is attributed to the formation of complexes with the two extractants. Different positive synergetic coefficients are gathered in Table 2 showing that whatever the molar ratio, a positive synergetic coefficient was obtained.
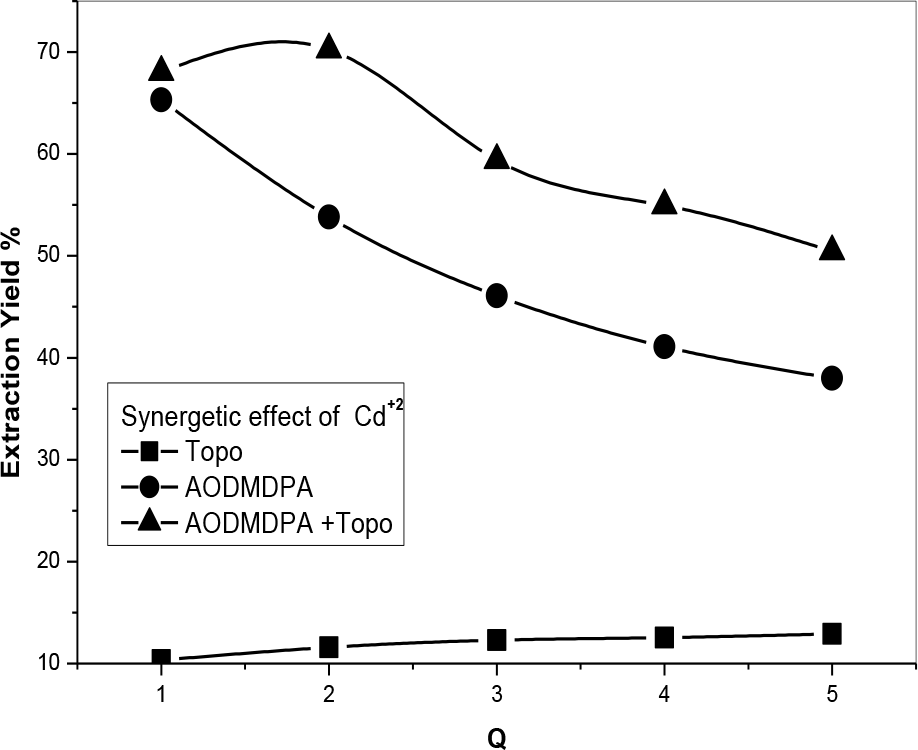
[Cd2+] = 5 × 10−4 M, (Vaq/Vorg) = 1; (AODMDPA: TOPO = 3: 1), T = 25°C.
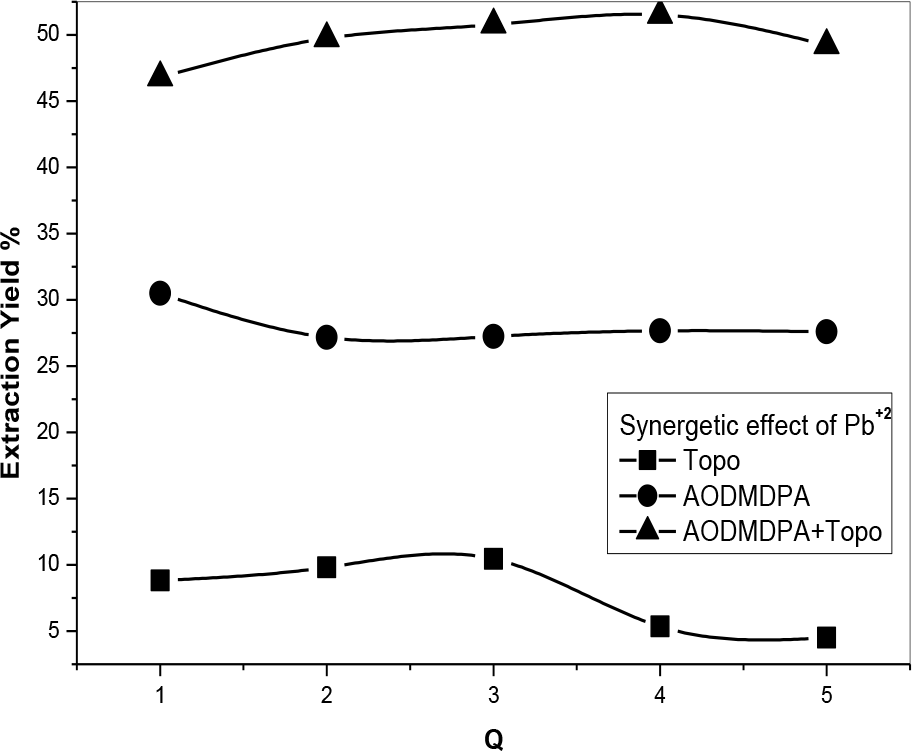
[Pb2+] = 5 × 10−4 M, (Vaq/Vorg) = 1;(AODMDPA: TOPO = 3: 1), T = 25°C.
Q: molar ratio Q1: Q = 1, Q2: Q = 2, Q3: Q = 3, Q4: Q = 4, Q5: Q = 5.
D1: Distribution factor using TOPO alone. D2: Distribution factor using AODMDPA alone.
S: Synergetic factor .
A new phosphonic acid extractant named aminooctylphosphonic acid easily synthesized under microwave using low-cost chemicals. Complete achievement of Cd2+ can be reached when Pb2+ extraction reaches 73% after two cycles. A positive synergetic effect was observed at an AODMDPA/TOPO ratio of 3. It is a very encouraging result that can lead us to recover Cd2+ and lower the concentration of Pb2+ from wastewater.
Open Science Framework: Spectrophotometric study of Solvent extraction of Pb (II) and Cd (II) by aminooctyldiphosphonic acid. https://doi.org/10.17605/OSF.IO/FMHT4.27
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Solvent extraction, Metal separation, Separation engineering, Metal-organic framework, Heterocyclic compounds
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Nov 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)