Keywords
Brainstem, early markers, neuron damage, protofibrils, rotenone, total glutathione
This article is included in the Research Synergy Foundation gateway.
Brainstem, early markers, neuron damage, protofibrils, rotenone, total glutathione
Glutathione (GSH) is a tripeptide of glutamic acid, cysteine, and glycine. GSH functions in cells as a cellular antioxidant, a cofactor of glutathione peroxidase, and a redox system regulator. Another role of GSH is in protecting cells, especially from toxic substances.1 GSH can conjugate to a protein and protect it from oxidation.2-4 GSH is also involved in detoxification in phase II drug metabolism.5 Most GSH is present in its reduced thiol tripeptide form, whereas the rest is oxidized as glutathione disulfide (GSSG). The equilibrium between GSH and GSSG is a function of the redox status of a cell, and specifically, the ratio of GSSG to GSH is an index of oxidative stress. It can therefore be said that the redox status is inversely related to the oxidative stress index. The sum of reduced GSH and oxidized GSSG is referred to as “total glutathione”.5-8 The concentration of total glutathione describes the capacity of the redox state in the cell. An increase in concentration of total glutathione indicates an increased ability of a cell to scavenge free radicals and protect the redox system, thereby protecting the cell from exposure to toxins.9,10 The typical concentration of GSH in a cell is 1–2 mM, except in hepatocytes, in which the concentration can be as high as 10 mM.9 In general, the more active a cell is, the higher the concentration of GSH is in that cell, and vice versa. In unhealthy cells, the concentration of GSH tends to be lower than in their healthy counterparts.9
Neurons are highly active cells and are responsible for up to 20% of the body's oxygen consumption, despite the brain being only 2% of body mass.2 Neuronal activities cause an increase in the concentration of reactive oxygen species (ROS), such as hydrogen peroxide, which is strongly oxidizing and thus harmful to cells.10 Physiologically, hydrogen peroxide is produced in the processes of signal transduction, oxidative stress, growth, cell proliferation, and apoptosis.11,12 The enzyme glutathione peroxidase, in the presence of its cofactor GSH, catalyzes the breakdown of hydrogen peroxide into water and oxygen. This is part of the system of redox equilibrium in a cell.4 If the redox equilibrium is perturbed, cells are said to be under oxidative stress and this causes biomolecules, such as proteins, to be oxidized.13,14 In neurons, one such protein is alpha-synuclein, and changes in its structure resulting from oxidation create insoluble protofibrils.15,16
Insoluble protofibrils tend to undergo aggregation in cytoplasm because cytoplasm is 70% water. Protofibrils are toxic, and the mechanism of the cell to prevent the toxicity is to form inclusion bodies.17 In the pathogenesis of neurodegenerative diseases, protofibrils are the precursors of Lewy bodies in Parkinson's disease and senile plaques in Alzheimer's disease.15
The process of protofibril formation and their deposition in cells and tissues varies between the neurodegenerative diseases.1 In Parkinson's disease, one of the causative factors for the formation of protofibrils from alpha-synuclein is exposure to neurotoxic pesticides, which induces oxidative stress pathways. The formation of protofibrils from alpha-synuclein can be prevented if cellular antioxidant activity is high, that is, if the concentration of GSH is high.18
Studies on GSH are numerous, particularly those examining the role of GSH as a redox buffer in cells, and also as a cellular and extracellular antioxidant nutritional supplement.1 Nevertheless, studies related to total glutathione are scarce and the importance of total glutathione in neurons is unclear. In this study, the total glutathione concentration in the brainstems of rats treated with rotenone was analyzed. Rotenone (a natural pesticide from the root of Derris sp.) is lipophilic and readily penetrates cell membranes,19,20 causing neurons to undergo oxidative stress.8 The aims of this research were to determine the total glutathione concentration in the upper and lower brainstems of Wistar rats treated with rotenone, and whether the sub-chronic exposure of rotenone causes the formation of protofibrils that, in humans, leads to the formation of Lewy bodies, as occurs in Parkinson's disease. The results reinforce the concept of pathogenesis of sporadic Parkinson's disease in rats treated with rotenone.
Seventy-two male Wistar rats aged 8–9 weeks and weighing 200–250 g were selected randomly from the animal house at SITH ITB. Breeding, maintenance, treatment, and sacrifice protocols were adapted to the provisions of the Health Research Ethics Committee, Faculty of Medicine, UNPAD–RSUP Dr. Hasan Sadikin Central General Hospital, Bandung, Indonesia (214/FKUP-RSHS/KEPK/Kep./EC/2010).8
Rotenone (R8875), sunflower seed oil (S5007), 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB, D8130), reduced L-glutathione (G4251), glutathione reductase (G3664), NADPH (N5130) and thioflavin S (T1892) were purchased from Sigma–Aldrich or Merck.
The selected rats were divided equally into two studies—determination of total glutathione and protofibril examination. Four independent variables were examined: 1) rat treatment group: blank (not treated), solvent-rotenone (intraperitoneally administered sunflower seed oil at a dose of 1 mL/kg body weight/day),8,21 and rotenone (intraperitoneally administered rotenone at a dose of 2.5 mg/kg body weight/day) groups18; 2) duration of rotenone treatment to examine subacute and subchronic exposure (9, 19 and 28 days); 3) observation time (days 10, 20, 30, 40, 50, and 60); and 4) the location in the brainstem (upper and lower brainstem).22 The concentrations of total glutathione and protofibrils were dependent variables (https://figshare.com/articles/dataset/Arrive-ten-aby/16880536).
Thirty-six male rats weighing more than 200 g were sacrificed by decapitation.23 The brainstems were isolated and separated between the upper and the lower regions and stored in liquid nitrogen. Samples were thawed at 4°C and washed three times with phosphate-buffered saline (PBS) containing 0.16% heparin, then homogenized using a homogenizer. The samples were diluted with 0.1 M phosphate buffer (pH 7) to a volume of 2 mL.21 Homogenates were centrifuged at 14,000 rpm for 10 min at 4°C. The obtained supernatant was stored as aliquots at −80°C.
The total glutathione measurement is defined as the concentration of GSH after glutathione reductase and NADPH are added to ensure all glutathione exists in the reduced form. In brief, the supernatant was thawed at 4°C, treated with 25% trichloroacetic acid and then centrifuged at 2000 × g for 15 min. Another supernatant was added GSH reductase and NADPH with the corresponding volume. DTNB was added and the absorbance of the solution was measured at λ = 412 nm (the by-product formed from the reaction of GSH and DTNB is yellow in solution) using a UV–vis spectrophotometer.8
Thirty-six male rats were sacrificed according to the cardiac perfusion method using ketamine (90mg/kg body weight) and xylazine (10 mg/kg body weight) administered by intraperitoneal injection (27-gauge needle and 1-mL syringe). A cardiac perfusion with ice-cold PBS was performed followed by perfusion of a 4% paraformaldehyde solution containing 0.1% glutaraldehyde. The brainstem was isolated, and the lower and upper brainstems were separated before being immersed into the fixative for less than 48 h, then embedded in a paraffin block. To facilitate embedding, first the brainstem specimens were dehydrated in ethanol at increasing concentrations (30%, 50%, 70%, 80%, 96%, and 100%). Then, the specimens were cleared using a toluene solution and infiltrated using a toluene/paraffin solution (1:1 v/v). Finally, the specimens were embedded in a paraffin block.24
Slicing was performed at the Histopathology Laboratory, Faculty of Veterinary Science, Bogor Agriculture Institute, Bogor (Indonesia). Both serial coronal sections of the upper brainstem and longitudinal sections of the lower brainstem were made with thicknesses of 4 μm. Based on a previous study, 10 serial sections showed no differences in histological profile. Thus, the serial sections 1–10, 11–20, 21–30, 31–40, 41–5021,25 were stained using thioflavin S.26
Preserved samples were deparaffinized using xylol followed by rehydration using ethanol, and the preparations were immersed into PBS solution containing 0.01% thioflavin S for 10 min. Protofibrils were identified using fluorescence microscopy and quantified using an unbiased stereological method.27 These experiments were repeated on days 20, 30, 40, 50, and 60.8
The protofibrils in brainstem slices were stained with thioflavin S and their density was measured by using a fluorescence microscope (Nikon Eclipse E800) equipped with a Nikon DXM1200F digital camera with a 450–550 nm filter. Yellow-green luminescence reveals the presence of protofibrils.28 The intensity of the emission was calculated using ImageJ 1.53a (NIH; Figure 1).29
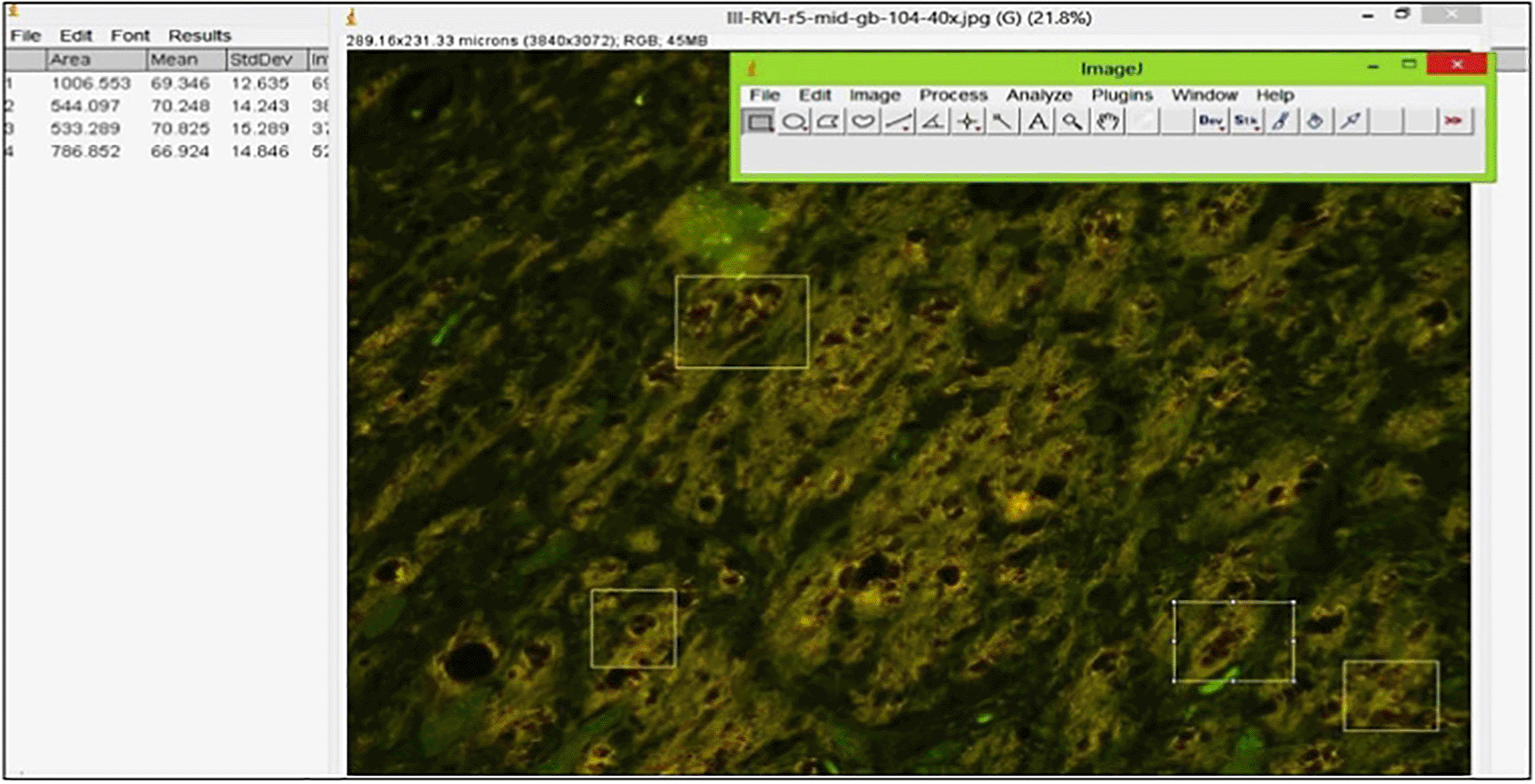
The brainstems of Wistar rats were stained using thioflavin S and observed using a fluorescence microscope at 400× magnification. Protofibrils were identified by yellow-green fluorescence, the intensity of which was determined by corrected density.
The average concentrations of total glutathione in the upper brainstems of Wistar rats in the blank, solvent-rotenone, and rotenone-treated groups were 7.78 ± 1.44, 7.05 ± 1.57, and 7.58 ± 1.32 μmol/mg brain wet weight, respectively (Figure 2). Total glutathione concentration was at its highest on day 20 in the blank group and on day 50 in the rotenone-treated rats. The lowest total glutathione concentrations were measured on days 10 and 20 in the blank and rotenone groups, respectively. In the lower brainstems of Wistar rats, the average concentrations of total glutathione in the blank, solvent-rotenone, and rotenone groups were 8.37 ± 0.73, 6.90 ± 1.14, and 5.77 ± 0.79 μmol/mg brain wet weight, respectively (Figure 3). The highest total glutathione concentration in the blank group was measured on day 50, and on day 10 in the rotenone-treated rats. The lowest total glutathione concentrations were measured on days 30 and 50 in the blank and rotenone groups, respectively.40 Total glutathione was different in the treated rats, especially in the rotenone group, albeit not statistically significant (p: 0.084). With respect to observation time, the concentration of total glutathione was not statistically significant (p: 0.608). Differences between upper and lower brainstems were also not significant (p: 0.372), although the concentration of total glutathione was higher in the upper brainstem.
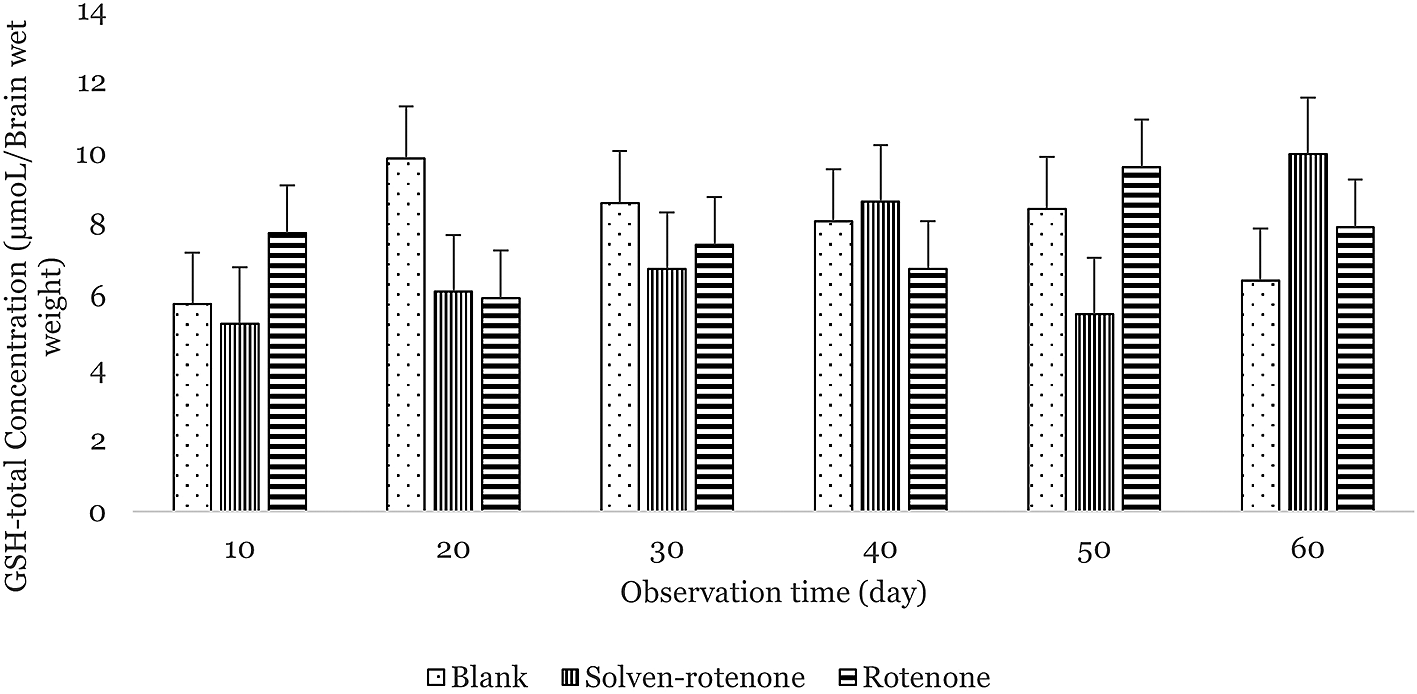
The concentration of total glutathione in the blank group (dots) was highest on day 20. The concentration of total glutathione in the solvent-rotenone group (vertical lines) was highest on day 60. The concentration of total glutathione in the rotenone group (horizontal lines) was highest on day 50.

The concentration of total glutathione in the blank group (dots) was highest on day 50 after acute exposure. The concentration of total glutathione in solvent-rotenone group (vertical lines) was highest on day 40. The concentration of total glutathione in the rotenone group (horizontal lines) was highest on day 10 after acute exposure.
To examine the existence of protofibrils, the sliced brainstem was immersed into a solution of thioflavin S and observed using a fluorescence microscope at 400x magnification. The average densities of protofibrils in upper brainstems in blank, solvent-rotenone, and rotenone-treated rats were 2076.45 ± 144.14, 2538.11 ± 224.96, and 2784.71 ± 289.98 protofibrils per μm2, respectively (Figure 4). The highest protofibril densities in the blank and rotenone-treated groups were measured on day 50 (Figure 5).
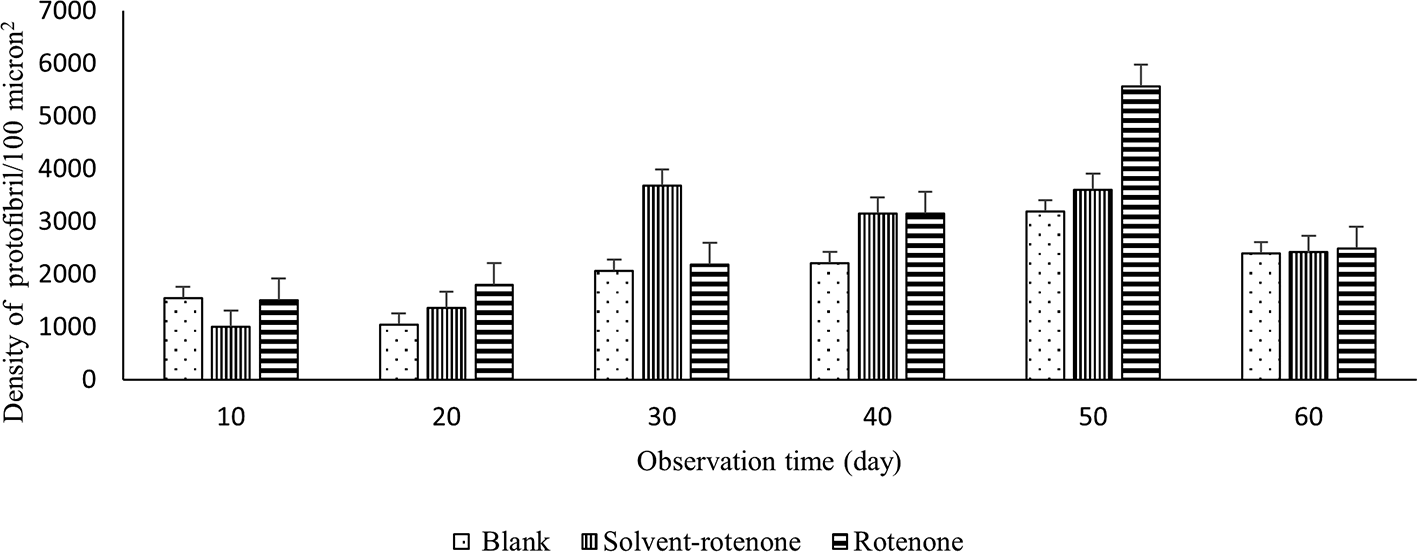
The protofibril density in the blank group (dots) was highest on day 50, the solvent-rotenone group (vertical lines) was highest on day 3, and in the rotenone group (horizontal lines) on day 50.

Observed at 400× magnification, yellow-green fluorescence indicates the presence of protofibril (black arrows). The highest protofibril densities were found in the blank group (A), solvent-rotenone group (B), and rotenone group (C) at days 50, 30, and 50, respectively.
https://figshare.com/articles/figure/Protofibril-ABY/16879819.
In the lower brainstem of rats in the blank, solvent-rotenone, and rotenone groups, the average protofibril densities were 2844.92 ± 220.98, 2931.85 ± 224.96, and 3122.08 ± 229.23 protofibrils per μm2, respectively (Figure 6) (https://doi.org/10.6084/m9.figshare.16528659). The highest protofibril density in the blank group was measured on day 50, and on day 30 in the rotenone group (Figures 6 and 7). Based on ANOVA, the differences in protofibril density within the observation time and treatment groups were significant (p: 0.001), as were differences between the upper and lower brainstem (p: 0.001). The protofibril density was higher in the lower brainstem than in the upper region

The protofibril density in the blank group (dots) was highest on day 50, and that in the solvent-rotenone group (vertical lines) was highest on day 30. The highest protofibril density in the rotenone group (horizontal lines) was measured on day 30.

Observed at 400× magnification, yellow-green fluorescence indicates the presence of protofibril (black arrows). The highest protofibril densities were found in the blank group (A), solvent-rotenone group (B), and rotenone group (C) at days 50, 30, and 30, respectively.
https://figshare.com/articles/figure/Protofibril-ABY/16879819.
GSH is a cellular antioxidant that acts as a free-radical scavenger. It is produced as a metabolic by-product in mitochondria. An increase in cell activity increases the production of free radicals, particularly ROS.1 The concentration of total glutathione indicates the ability of a cell to prevent the harmful effects of free radicals. Brain oxygen consumption is higher than in other organs, so ROS levels are typically higher in the brain. Conversely, the concentration of GSH is relatively lower in the brain than in other organs, such as kidney and liver. A decrease in GSH levels in the brain will cause the level of oxidative stress to increase, resulting in lipid oxidation that can cause neuronal death.30 Depletion of the total glutathione in the substantia nigra is indicative of neurodegeneration, such as in Parkinson's disease.31 Pharmacokinetically, GSH plays a role in phase II drug metabolism, becoming sequestered by conjugation through its thiol group such that it cannot traverse the cell membrane. An interaction between rotenone and GSH has not been reported. Previous studies have suggested that rotenone inhibits mitochondrial complex I, which decreases GSH concentration,7,8 indicating that cellular free radicals increase in abundance, resulting in oxidative stress. This condition can be prevented by monitoring the changing concentration of total glutathione.
The brainstem was selected for study as it is closely associated with neurogenerative diseases, particularly sporadic Parkinson's disease. The pathogenesis of Parkinson's disease originates from damage to the 10th cranial nerve (vagus nerve) where its pathway crosses the brainstem. Thus, measuring the total glutathione in the brainstem might be an initial indicator of neurodegenerative disease. In the present study, the concentration of total glutathione in the upper brainstem was higher than in the lower brainstem. This might indicate that neuronal damage had occurred in the lower brainstems of these rats.22
The concentration of total glutathione in the upper brainstems of rotenone-treated rats on day 60 was higher than that in the blank group. The concentration of total glutathione in the upper brainstems of rats treated with rotenone was 7.58 μM and that in the lower brainstem was 5.77 μM. To make the comparison with humans, these concentrations can be multiplied by a factor of 67.30,31 This estimates the concentrations in humans to be 0.5 mM (upper brainstem) and 0.38 mM (lower brainstem). In humans, the normal concentration of total glutathione is in the range 1–2 mM.1,30 Therefore, we conclude that sub-chronic exposure to rotenone decreases the concentration of total glutathione in brainstem.
The concentration of total glutathione in all the treatment groups fluctuated over time. This indicates that the homeostasis of neurons remained stable. However, our data indicates that rotenone exposure causes the concentration of total glutathione to decline. Thus, it might be predicted that GSH could be used as an indicator of exposure to a specific toxic substance that induces oxidative stress in neurons. Meanwhile, the concentration of GSH was obtained from 60% of total glutathione.32 Applying this to our results, the concentrations of GSH in the rotenone-treated rats were 4.5 μM (upper brainstem) and 3.4 μM (lower brainstem).
A reduction in GSH concentration causes oxidative stress resulting in the oxidation of intracellular biomolecules, such as proteins, lipids, and nucleic acids. In neurons, alpha-synuclein protein is readily oxidized. The physiological role of this protein is unknown, but it is well known that oxidation due to neurotoxic exposure results in structural changes that cause aggregation and formation of protofibrils.15 Thus, the measurement of protofibrils might serve as a marker of neurotoxic exposure, in this case to rotenone that causes oxidative stress in neurons.
Proteins can function as receptors, enzymes, or transporters, and the structure of a protein determines its function, particularly water-soluble proteins.14 If a protein is oxidized, its structure can change. Water-soluble proteins can become insoluble and aggregate. Such aggregates can form inclusion bodies for 20 days after exposure to a neurotoxin.33,34 Inclusion bodies can spread to other parts of the brain and cause clinical symptoms. In this study, the sub-chronic exposure of a neurotoxin was repeated daily for 9, 19, and 28 days. These rats were observed every 10 days for 60 days. Over this time course, the concentration of total glutathione fluctuated and tended to decrease, whereas protofibril density tended to increase. There is a weak correlation between the increase of total glutathione and the formation of protofibrils at 0.3 (sig: 0.001). Furthermore, the formation of protofibrils can influence neuron activities. Protofibrils formed in neurons can cause damage that ultimately leads to cell death.35,36 These dead cells cannot be replaced with new neurons, so the size of the brain will decrease.37
The measured protofibrils are likely alpha-synuclein protein aggregates, although only a specific labelling protocol would confirm this. The density of protofibrils in brainstem tends to increase over the time course of observation, indicating an increase in protein aggregation, particularly in rotenone-treated rats. The lower brainstem is more abundant with protofibrils than the upper brainstem. This is consistent with the pathogenesis of those neurodegenerative diseases38 that originate from damage to the 10th cranial nerve, which is followed by specific clinical symptoms, particularly gastrointestinal tract disorders. The pathway of the 10th cranial nerve starts in the lower brainstem and travels to the upper brainstem in the substantia nigra.22 If dopaminergic neurons are damaged, symptoms of Parkinson's disease present. This also applies to Alzheimer's disease, in which the protein beta-amyloid forms aggregates in any part of the brain.38,39
Thioflavin-S-labeled protofibrils were measured by their fluorescence intensity.29 Image analysis showed that the density of protofibrils in the lower brainstem is higher than that in the upper region. Protofibrils were also distributed around the neurons and extracellular matrix, specifically in neurofibrils (Figures 5 and 7). To conclude, the process of neurodegeneration can be prevented by avoiding oxidative stress condition.
In this study, we propose measurement of total glutathione for identifying the capacity of neurons to avoid oxidative stress. If the concentration of total glutathione is high, neurons are in a homeostatic condition and have the ability to self-recover by shifting the steady state of the redox system. Therefore, water-soluble proteins are not oxidized and do not form protofibrils. The development of GSH as the indicator of oxidative stress cannot be applied in humans. Other studies are investigating alpha-synuclein in erythrocytes. It is hoped that a marker for the early detection of neurogenesis process could be developed, and thus, neurodegenerative diseases might be slowed or even prevented as early as possible to improve quality of life.
Rotenone exposure subcortically causes the concentration of total glutathione in brainstem to decrease and concentrations of total glutathione in the lower brainstem were lower than in the upper region. Conversely, protofibril density tended to increase with rotenone treatment. The concentration of total glutathione is inversely proportional to the density of protofibrils. Decreased GSH might be an early indicator of neuronal damage.
The author is Arief Budi Yulianti, from Medical biology Department, Faculty of Medicine, Universitas Islam Bandung (UNISBA), Indonesia. My contribution in this paper are in introduction, methods, result, and discussion.
The first co-author is Sony Heru Sumarsono, from Research Group of Physiology, Developmental Biology and Biomedical Science, School of Life Science and Technology, Institut Teknologi Bandung (ITB), Indonesia. His contribution in this paper are in introduction and discussion.
The second co-author is Ahmad Ridwan from from Research Group of Physiology, Developmental Biology and Biomedical Science, School of Life Science and Technology, Institut Teknologi Bandung (ITB), Indonesia. His contribution in this paper are in introduction and methods.
The third co-author is Ayda T Yusuf from Research Group of Physiology, Developmental Biology and Biomedical Science, School of Life Science and Technology, Institut Teknologi Bandung (ITB), Indonesia. Her contribution in this paper are in introduction, result and discussion.
Figshare: Glutathione total concentration in upper and lower rats Wistar brainstem treated rotenone, https://doi.org/10.6084/m9.figshare.16528659.
This project contains the following underlying data:
- Data-aby-gsh.xlsx (The data presented is total glutathione concentration in the brainstem.)
- Data-aby-protofibril.xlsx (The data presented is density of protofibrils in the brainstem.)
Figshare: Protofibril-ABY, https://doi.org/10.6084/m9.figshare.16879819.
This project contains the following underlying data:
The data presented is flouresence photomicrogrph brainstem with thioflavin staining.
Figshare: Arrive ten checklist-aby.pdf, https://doi.org/10.6084/m9.figshare.16880536.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
This article does not report studies involving human participants. Animals were handled according to the requirements of the Health Research Ethics Committee, Faculty of Medicine, UNPAD–RSUP Dr. Hasan Sadikin Central General Hospital, Bandung, Indonesia (214/FKUP-RSHS/KEPK/Kep./EC/2010).
This research was funded by a grant from the Faculty of Medicine, Universitas Islam Bandung. We are grateful to the Dean and staff of Universitas Islam Bandung for supporting this research, to the Rector and staff of Universitas Islam Bandung who led the Paper Camp activities, and also to Research Gate Fondation (RSF) who facilitated the writing of this paper.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Parkinson's disease, Rotenone, alpha-synuclein, tyrosine hydroxylase.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuronal glutathione and neurodegenerative disorders
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Nov 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)