Keywords
bleeding, cancer, factor Xa inhibitor, oral anticoagulant, venous thromboembolism.
bleeding, cancer, factor Xa inhibitor, oral anticoagulant, venous thromboembolism.
Cancer patients are five times more likely to experience venous thromboembolism (VTE) than the general population.1 Second only to cancer itself, VTE is the second most common cause of mortality in cancer patients.2 According to previous clinical management recommendations, the typical VTE treatment in cancer patients involves the initial use of parenteral low-molecular-weight heparin (LMWH) followed by long-term use of oral vitamin K antagonists (VKA).3 However, recent recommendations proposed factor Xa inhibitors as one of the options of the main initial treatment for VTE.4
Factor Xa inhibitors are preferred over LMWH and VKA because they conveniently do not require injections every day compared to LMWH, their more predictable effects, lack of monitoring or frequent repeat doses, and fewer drug interactions compared to VKA.5 An earlier systematic review reported differences between factor Xa inhibitors and standard anticoagulation drugs in the incidence of recurrent VTE and major bleeding.6 Based on this research, the present meta-analysis aims to evaluate the effectiveness of factor Xa inhibitors for the management of venous thromboembolism, particularly in patients with cancer.
We adopted the Preferred Reporting Items for Reviews and Meta-Analyses guidelines for analysis reporting.7 Any RCTs that studied VTE rates or major bleeding, as primary or secondary outcomes, in cancer patients who received an oral factor Xa inhibitor were included. Phase II trials, trials with an antiplatelet control group, and trials using an anticoagulant as VTE post-procedure prophylaxis were excluded.
We conducted a systematic search using the PubMed and Cochrane Library databases on April 24, 2020, after gaining approval from the Institutional Review Board. As for the title, abstract, and medical subject heading, we used search terms like “cancer,” “factor Xa inhibitor,” “oral anticoagulant,” “venous thromboembolism,” “apixaban,” “rivaroxaban,” “edoxaban,” “prophylaxis,” “bleeding,” “thromboembolism,” “thromboprophylaxis,” “randomized,” and “rct.”
We screened more studies by looking at the references in the included articles. Two investigators independently selected studies, with disagreements resolved through discussion and a third investigator's opinion. Thereafter, for each report, two investigators independently extracted the following information: authors, year of publication, trial name, cancer status, sample size, dose and duration of anticoagulation, duration of patient follow-up, and outcomes for the two treatment groups where available.
We determined four comparison groups: (1) factor Xa inhibitor versus LMWH as treatment for VTE; (2) factor Xa inhibitor versus VKA as treatment for VTE; (3) factor Xa inhibitor versus placebo as prophylaxis for VTE; (4) factor Xa inhibitor versus VKA as prophylaxis for VTE. The outcomes of our meta-analysis were recurrent VTE or new VTE event rates and incidence of major bleeding. VTE events were confirmed by leg vein ultrasound scanning, D-dimer testing, or both; alternatively, clinically overt pulmonary embolism was confirmed by imaging. Major bleeding was defined as in Schulman et al.8
The Cochrane Collaboration Risk of Bias Tool was used by two independent investigators to assess the methodological quality of included studies, and the GRADE approach was employed to grade each outcome.9,10 Any disputes were settled through discussion with a third investigator. We calculated odds ratios (ORs) for all outcomes at the longest follow-up period and used Review Manager (RevMan v5.3 2014) to apply the Mantel−Haenszel fixed-effects method. We conducted a modified intention-to-treat analysis including patients who had received ≥ 1 medication dose. We planned to a conduct sensitivity analysis by removing studies likely to be biased. The I2 statistic was used to assess statistical heterogeneity between studies. If the heterogeneity was > 50%, we applied a random-effects model for analysis.11
The search identified 202 citations in PubMed and 41 in the Cochrane Library, among which 43 were duplicates (Figure 1). We found 22 more studies of which we evaluated the full text. Four studies were post-procedure prophylaxis trials, three lacked a control, two were phase II trials, and two were extensions of included trials, so 11 were omitted. As a result, we could include 11 studies in our analysis.12–22
Table 1 lists the characteristics of the included studies. There were four trials on apixaban, four on rivaroxaban, and three on edoxaban. The study size ranged from 300 to 1,170 patients. Five studies were subgroup analyses of patients with cancer from larger primary trials.12–16 We pooled their data only from the subgroup of patients with cancer, not all study population. One study was a pooled analysis of the subgroup of patients with cancer in “sister” trials.17 Four trials13,18–20 compared factor Xa inhibitors with LMWH, and three12,14,17 compared factor Xa inhibitors with VKA as a VTE treatment. Two trials15,16 compared factor Xa inhibitors with placebo and two21,22 compared factor Xa inhibitors with VKA as prophylaxis of VTE. We included one trial that investigated two doses of edoxaban for VTE prophylaxis, where the outcomes of both groups were combined and analyzed as one intervention group.16
The risk of bias across domains is presented in Figure 2. In most studies, the randomization process, adherence to the intervention, assessment, missing outcome results, and reporting were deemed adequate. In four trials, participants were blinded. The percentage of patients not followed up ranged from 0.2% to 5.6%. All trials reported the results from modified intention-to-treat analysis.
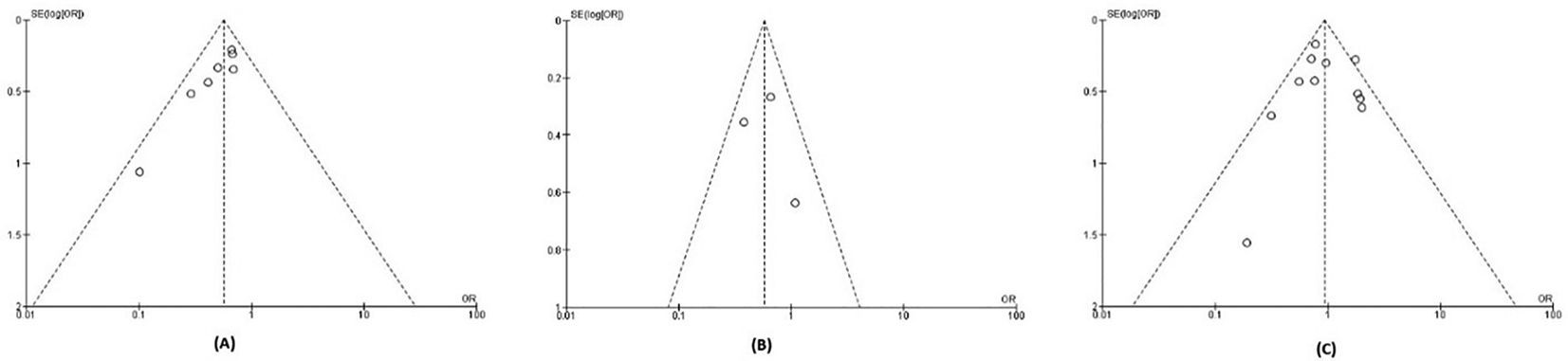
The quality of evidence for each outcome analyzed using the GRADE approach is presented in Table 2. We did not downgrade from the risk of bias, inconsistency, indirectness, and imprecision aspect of all outcomes, because of a low risk of bias, no substantial heterogeneity, a large enough sample size, and narrow confidence interval (CI). We downgraded one level for the major bleeding outcome because the funnel plot of major bleeding outcome suggested publication bias (Figure 3).
Seven studies involving 4,771 patients reported VTE recurrence (Table 2). Recurrence occurred in 4.9% (117/2,399) of patients allocated to factor Xa inhibitors, 9.1% (132/1,445) allocated to LMWHs, and 6.9% (64/927) of those allocated to VKAs. In comparison (Figure 4), the reduction of the risk of VTE recurrence with factor Xa inhibitors compared to LMWH was acceptable (four trials; OR 0.60; 95% CI 0.45–0.80; P < 0.01), without substantial heterogeneity (I2 = 26%; P = 0.26). VTE recurrence rates were lower in patients treated with factor Xa inhibitors compared to patients treated using VKAs (three trials; OR 0.51; 95% CI 0.33–0.78; P < 0.01), without substantial heterogeneity (I2 = 0%; P = 0.37).
Three studies, including 2,056 patients, reported the incidence of new VTE after anticoagulant prophylaxis. The factor Xa inhibitor group had a 4.1% (42/1,021) VTE occurrence rate, while the VKA and placebo groups each had 1.45% (5/355) and 9.6% (65/680), respectively. According to the meta-analysis shown in Figure 5, there were similar VTE incidences in the factor Xa inhibitor and the VKA groups (one trial; OR = 1.08 [95% CI, 0.31–3.77]; P = 0.90); however, the heterogeneity analysis could not be applied. The estimated effect of factor Xa inhibitors on VTE incidence compared to placebo showed a statistically significant reduction (two trials; OR = 0.54 [95% CI, 0.35–0.81]; P < 0.01), without substantial heterogeneity (I2 = 31%; P = 0.23).
Eleven studies, including 7,965 patients, reported major bleeding (Table 2). Major bleeding occurred in 5.5% (231/4,178) of patients allocated to factor Xa inhibitors, 3.6% (52/1445) to LMWHs, 8.1% (134/1,662) to VKAs and 1.3% (9/680) to placebo. According to the meta-analysis shown in Figure 6, the acceptable increase of risk cannot be confirmed from the description of major bleeding with factor Xa inhibitors compared to LMWH, as based on an OR of 1.34 (95% CI, 0.83–2.18) with a P = 0.23, which is not statistically significant. However, factor Xa inhibitors significantly reduced the risk of major bleeding compared to VKAs (five trials; OR = 0.71 [95% CI, 0.55–0.92]; P = 0.009), without substantial heterogeneity (I2 = 0%; P = 0.72). The risk of major bleeding was higher with factor Xa inhibitors versus placebo (two trials; OR = 1.98 [95% CI, 0.88–4.44]; P = 0.10) but not statistically significant, without substantial heterogeneity (I2 = 0%; P = 0.96).
The aim of this meta-analysis was to determine the efficacy and safety of factor Xa inhibitors for VTE treatment in cancer patients. Recurrence was 4.9%, 9.1%, and 6.9 % for the factor Xa inhibitor, LMWH, and VKA groups, respectively. All were lower than the findings of a retrospective cohort study which reported an incidence of 13.1%, 17.6%, and 17.9%, respectively.23 Our review of four studies involving over 4,771 patients found that factor Xa inhibitors were associated with a lower risk of VTE recurrence when compared to LMWH, and even lower when compared to VKA. This result was consistent with a recent meta-analysis which combined data from RCTs and retrospective cohort studies.24
Another finding in our meta-analysis in terms of safety profiles was that factor Xa inhibitors were associated with an increased risk of bleeding when compared to LMWH, but a lower risk when compared to VKA. This result is in line with the findings of other systematic reviews.24–26 However, another meta-analysis found a significantly higher incidence of bleeding (two trials, OR= 2.72 [95% CI: 1.05–7.01]; P= 0.039) with factor Xa inhibitors, relative to LMWH.27 Importantly, the bleeding outcome in comparison to LMWH was the result of pooled data from nonspecific cancer patients. The results of the analysis of major bleeding in comparison to LMWH were mainly influenced by those of the HOKUSAI VTE Cancer trial and the recent CARAVAGGIO trial.28,29 Both had different results: the former showed significantly higher bleeding in the edoxaban group while the second showed similar major bleeding events between groups.
Our meta-analysis also provided information about the efficacy of factor Xa inhibitors as prophylaxis, which suggested that, compared to placebo, it can significantly reduce VTE incidence. According to a recent clinical practice guideline, high-risk cancer outpatients can receive thromboprophylaxis with a factor Xa inhibitor or LMWH, in the absence of major risk factors for bleeding.30 The high cost and the pain of daily LMWH injections was avoided with the factor Xa inhibitor regimen.
With respect to factor Xa inhibitors and LMWH, the inclusion of the CARAVAGGIO trial, with highly rigorous evidence, increased the accuracy of the estimated outcomes. There are a number of limitations to the current meta-analysis: the majority of the data corresponded to subgroup or post-hoc analyses. Further, the following variables were not controlled for: cancer stage, type of cancer, follow-up period. While most of the included studies evaluated patients for six months, the optimal duration of anticoagulation treatment was not evaluated to achieve an agreement. Finally, despite our systematic electronic database search and our investigation of the references in the included studies, we may have missed relevant studies.
Factor Xa inhibitors are effective for VTE management in patients with cancer; however, they are also associated with an increased bleeding risk compared to LMWH, but decreased when compared to VKA.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: PRISMA checklist for ‘Factor Xa inhibitor for venous thromboembolism management in Patients with cancer: a systematic review and meta-analysis’. https://doi.org/10.6084/m9.figshare.16590086.v331
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This work was performed as part of Johanes Nugroho employment at the Department of Cardiology and Vascular Medicine, Universitas Airlangga/Dr. Soetomo General Hospital, East Java, Indonesia. The authors also would like to thank Enago (www.enago.com) for the English language review and this work was supported by the Indonesian Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: cancer, biostatistics
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiovascular Medicine and Intervention
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 08 Dec 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)