Keywords
Transthyretin cardiac amyloidosis, Aortic stenosis, Transcatheter aortic valve replacement, Ventricular fistula
Transthyretin cardiac amyloidosis, Aortic stenosis, Transcatheter aortic valve replacement, Ventricular fistula
AS: aortic stenosis
ATTRwt: wild-type transthyretin cardiac amyloidosis
CA: cardiac amyloidosis
CTS: carpal tunnel syndrome
HF: heart failure
RV: right ventricular
TAVR: transcatheter aortic valve replacement
There is a high prevalence of wild-type transthyretin cardiac amyloidosis (ATTRwt) among patients with degenerative aortic stenosis (AS). We describe the first case of an aortic to right ventricular (RV) fistula after transcatheter aortic valve replacement (TAVR).
An 88-year-old Caucasian, retired, male patient was referred for transcatheter aortic valve replacement (TAVR) due to degenerative severe AS and heart failure (HF). He was first evaluated in 2015, following the diagnosis of atrial fibrillation and a systolic murmur.
Regarding his past medical history, he was a former smoker and had known dyslipidemia. Additionally, he had undergone bilateral carpal tunnel syndrome (CTS) intervention in 2015 and 2016.
On top of moderate-to-severe aortic stenosis (maximal mean gradient: 32 mmHg; area: 0.9 cm2), his first echocardiogram revealed mild left ventricular hypertrophy, preserved systolic function and a severely dilated left atrium.
Given his previous history of CTS, cardiac amyloidosis diagnosis workup was started. Plasma cell dyscrasia was ruled out and technetium-99m (99m-Tc) 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) scintigraphy showed an intense myocardial uptake, establishing a non-invasive diagnosis of concomitant ATTRwt, after excluding transthyretin (TTR) mutations.
At that time, the patient was in the New York Heart Association class II, denying angina or syncope but in 2016 he developed HF symptoms and required admission. During hospitalization, a new echocardiogram revealed mild systolic dysfunction (left ventricular ejection fraction (LVEF) 45%) and a stress echo was requested. Following dobutamine infusion, despite no cardiac output improvement, systolic function and mean transaortic gradient increased up to an LVEF of 59% and 40 mmHg, respectively, confirming the severity of AS.
In spite of the optimal medical therapy being used, HF decompensation recurred and TAVR was considered, so a computed tomography was performed to obtain different measurements including the aortic annulus: mean diameter (24.9 mm) and area (514 mm2).
The TAVR procedure was performed using femoral access and standard technique. Since previous aortic annulus measurement corresponded to lower limits for 29 mm Sapien 3 (Edwards) prosthesis and the aortic valve was a little calcified, that prosthesis was chosen and a balloon was inflated using 2 mL less than the nominal value. Although the TAVR was correctly implanted, during the intervention, the patient suffered a complete atrioventricular block requiring pacemaker implantation and an aortic to right ventricular (RV) fistula was observed by control angiography immediately after implantation (Figure 1).13
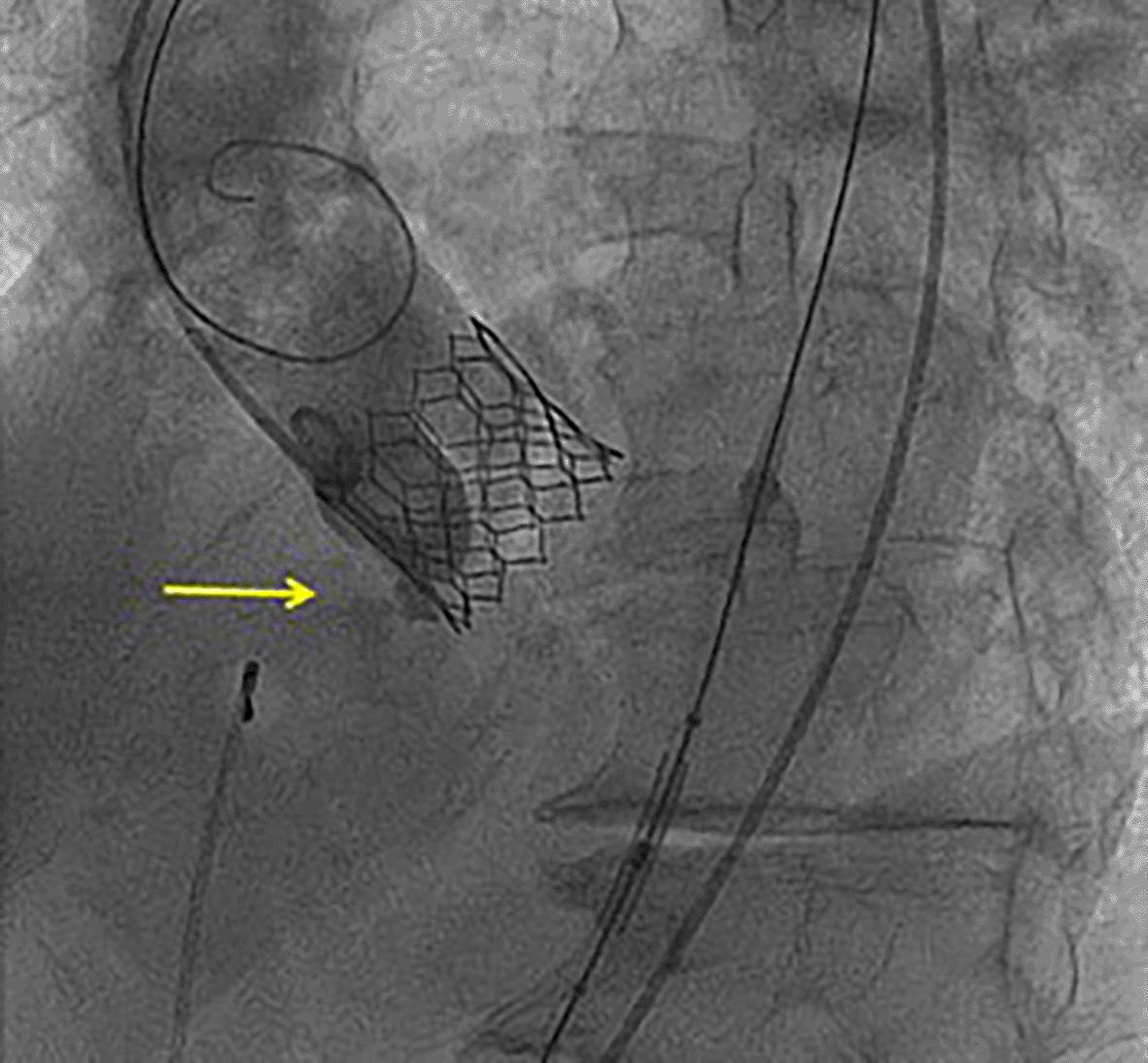
A conservative management style was implemented for further interventions, with imaging and clinical follow-up. The patient presented no complications during a 2-year-follow-up. Repeated transthoracic echocardiograms revealed stable aortic to RV fistula, without hemodynamic changes (Figure 2).14–18
AS is the most common valve disease in the elderly population. Nearly 5% of patients aged 75 years and over have at least moderate AS,1 with a prevalence of >4% in octogenarians.
Cardiac amyloidosis (CA) has been traditionally associated to a restrictive cardiomyopathy, caused by the extracellular deposition of proteins in the myocardium. Primary or amyloid light-chain (AL) amyloidosis and transthyretin cardiac amyloidosis (in its hereditary or wild-type (ATTRwt) forms) are the most common subtypes of CA. Recently, transthyretin amyloidosis (ATTR) has been considered much more prevalent than AL.2
Amyloid can infiltrate all components of the heart, from the conduction system to vessels. Amyloid infiltration has typically been associated with atrioventricular valve infiltration, but recently, the coexistence of AS and ATTRwt has emerged as a very prevalent clinical scenario.2
In 2016 a histological report revealed that occult ATTRwt had a prevalence of 5.3% among patients undergoing surgical aortic valve replacement due to severe calcific aortic stenosis. The subjects in the report were predominantly males, with a mean age of 75 years old.3
A higher prevalence was found later in screened populations undergoing TAVR as these patients tend to be older.3 An American study prospectively screened AS patients undergoing TAVR, using technetium pyrophosphate scintigraphy and found a prevalence of 16% among them.4 More recently, a European study observed that the combination of AS and amyloid is common and affects around one in eight elderly patients with severe AS being considered for TAVR.5 Therefore, clinical, ECG and imaging red flags for CA should be systematically searched for in patients with AS to identify concomitant ATTRwt.
Both entities, AS and ATTRwt, share a common demographic and clinical profile, being considered part of the aging process.6 There is increasing data pointing out to a causative link between them though.6 Oxidative stress, inflammation and extracellular remodeling may be involved in TTR amyloidogenic process6 and these factors are also a central part of AS’ pathophysiology. Thus, it is possible that amyloid deposits could be induced or accelerated in patients with AS.
The association between AS and CA is not just prevalent, but also dangerous. Some authors3,7 have described a higher mortality in patients with AS and CA compared to those with isolated AS, while in a recent cohort, mortality was not affected among those patients with ATTR and AS undergoing TAVR.8 The worse prognosis in these patients could be mainly caused by CA, even after valve replacement. To support this, the latest cohort was presented with an increase of HF admissions after TAVR.8
According to different groups,9,10 either repeated balloon valvuloplasties or TAVR is the best therapeutical options considering these patients’ frailty. Nowadays, TAVR is a procedure that commonly sees favorable outcomes, but some frequent complications (conduction disturbances, ...) might occur. Nonetheless, aortic to RV fistula formation is a rare complication (0.004% according to reported cases).11 This unusual complication does not often require repair and is reversible in most cases.
This case represents a typical example of diagnosis and management of AS and concomitant ATTRwt in an elderly patient. Low-flow, low-gradient AS has been shown to be a frequent form of AS presentation in ATTR.4 In this setting, the dobutamine stress test plays a crucial role in order to correctly evaluate AS’s severity and guide management. Attitude regarding AS in patients with CA should be personalized.
Our case illustrates a rare complication of an aortic to RV fistula. Although similar cases have been previously reported,11 to our knowledge, this is the first case of an aorto-RV fistula after TAVR in a patient with concomitant AS and ATTRwt. Aortic to RV fistula seems to be a rare complication, and cardiac amyloidosis was confirmed in just this case out of the four at our own center. Of note, it is the only one in which the fistula did not resolved during follow-up and we hypothesize its relationship with tissue’s fragility due to amyloid deposition.
The exact mechanism for fistula development after TAVR is not fully understood. Possible reasons to justify its development include congenital or acquired sinus of Valsalva aneurysms, trauma, or infections. Most cases of aortic to RV fistulas have been described in patients in whom a balloon-expanded transcatheter valve was used,11 possibly conditioned by trauma and oversizing. In this case, we believe that amyloid deposits on the aortic valve annulus might have led to a more friable substratum, making this case prone to complications, mainly complete AV block and aortic to RV fistula.
Several studies have found myocardial amyloid deposits in a significant percentage of patients with AS. Different authors have identified amyloid deposits in prosthetic valves explanted3 and in endomyocardial biopsies from basal left ventricle septum.3 Histological analysis of the interventricular septum performed by Moreno et al12 in a patient who developed a complete AV block after TAVR discovered two different potential mechanisms to explain the patient’s complication: a localized hematoma at the site of aortic valve prosthesis expansion, which could justify trauma damage on the conduction system; and amyloid deposits.
Generally, conservative management with annual re-evaluation is accepted in ventricular fistulas. Except when significant symptom development or hemodynamic instability occurs. Based on our own experience, a conservative approach is an adequate option, even in cases with concomitant ATTRwt.
1. To remind the importance of extensive clinical and imaging evaluation before transcatheter aortic valve replacement.
2. To emphasize the high prevalence of ATTRwt among patients with degenerative aortic stenosis undergoing TAVR and the need of amyloid screening in this clinical scenario.
3. To highlight the role of dobutamine stress echocardiogram in this setting.
4. To increase awareness about possible TAVR complications in patients with concomitant AS and ATTRwt and how to approach these cases in order to minimize them.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: Video 1: Aortic to right ventricular fistula after transcatheter aortic valve replacement observed by fluoroscopy, https://doi.org/10.6084/m9.figshare.17122124.v1.13
Figshare: Video 2: Aortic to right ventricular fistula after transcatheter aortic valve replacement. Control echocardiogram (subcostal view) during follow-up, https://doi.org/10.6084/m9.figshare.17122181.v1.14
Figshare: Video 3: Aortic to right ventricular fistula after transcatheter aortic valve replacement. Control echocardiogram (parasternal long axis view) during follow-up, https://doi.org/10.6084/m9.figshare.17122211.v1.15
Figshare: Video 4: Aortic to right ventricular fistula after transcatheter aortic valve replacement. Control echocardiogram (parasternal short axis view) during follow-up, https://doi.org/10.6084/m9.figshare.17122226.v1.16
Figshare: Video 5: Aortic to right ventricular fistula after transcatheter aortic valve replacement. Control echocardiogram (apical five chamber view) during follow-up, https://doi.org/10.6084/m9.figshare.17122241.v1.17
Figshare: Video 6: Aortic to right ventricular fistula after transcatheter aortic valve replacement. Control echocardiogram (zoom on apical five chamber view) during follow-up, https://doi.org/10.6084/m9.figshare.17122265.v1.18
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Written informed consent from the patient for the use and publication of the patient’s data was obtained.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Nitsche C, Scully P, Patel K, Kammerlander A, et al.: Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. Journal of the American College of Cardiology. 2021; 77 (2): 128-139 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: cardiovascular imaging, valvular heart disease, cardiac amyloidosis, coronary heart disease, cardiovascular magnetic resonance imaging.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 10 Dec 21 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)