Keywords
Rehabilitation device, Electrical stimulation, Obstructive Sleep Apnea, Genioglossus muscle, Laryngopharyngeal sensory test.
Rehabilitation device, Electrical stimulation, Obstructive Sleep Apnea, Genioglossus muscle, Laryngopharyngeal sensory test.
Sleep apnoea is a significant cardiovascular risk factor. Its consequences include systemic high blood pressure, pulmonary hypertension, myocardial infarction and cerebrovascular disease [(Caples et al., 2005), (Rangel-León et al., 2015)]. It also produces metabolic alterations related to the control of glycemia and lipids (Caples et al., 2005). Additionally, it is linked to obesity, which creates a vicious cycle: obesity causes sleep apnoea, and sleep apnoea worsens obesity by altering the response to hormonal mediators of satiety (Romero-Corral et al., 2010).
Despite representing a pathology with multiple complications, there are limited treatments for obstructive sleep apnoea-hypopnoea syndrome (OSA). The adherent use of continuous positive airway pressure (CPAP) has been shown to have beneficial effects on OSA outcomes, including avoiding further deterioration in the airway (Maurer et al., 2012); however, the adherence rate for this device is low (even less than 40% in some cases), forcing researchers to look for alternatives (Kezirian et al., 2010). Surgical procedures related to the treatment of OSA have variable results, with high mortality rates (Maurer et al., 2012) and effectiveness ranging from 20% to 60% (Maurer et al., 2012).
Recent research was investigated electrical stimulation of the upper airway in animals and humans with OSA. Animal studies have consisted of inserting needle electrodes orally into the genioglossus muscle of awake dogs or stimulating the hypoglossal nerve in anaesthetised and non-anaesthetised felines, checking the response of the airway muscles in stimulations with frequencies up to 50 Hertz (Hz) (Kezirian et al., 2010). Similarly, rats were stimulated on the upper laryngeal nerve with frequencies between 30 Hz and 40 Hz for 50 seconds, causing swallowing reflexes, which shows the effects of the action on the muscles (Tsuji et al., 2015).
Human studies for the management of OSA began with the intraoral electrical stimulation of the upper airways carried out by Guilleminault in the 1970s (Guilleminault et al., 1978). These early interventions did not detail the muscle groups on which electrostimulation was exerted, nor did they have diagnostic methods for determining whether the stimulation was taking place on dilating muscles or airway constrictors. Any of these reasons could explain a simultaneous stimulation of dilating and constrictor muscles of the airway, which could be responsible for the failure of such interventions.
Around ten years later, research focused specifically on the main dilating muscle of the airway, the genioglossus, showing that genioglossus stimulation reduced the apnoea–hypopnoea index (AHI) (Schwartz et al., 1993) and daytime sleepiness. However, the successful stimulation of upper airway muscles and relief from upper airway obstruction still has problems, such causing the patient to wake during sleep and the need to implant electrodes invasively on the hypoglossal nerve (Yoo & Durand, 2005).
More recently, a device has been developed that seeks to stimulate the hypoglossal nerve by electric current (Maurer et al., 2012). This device performs functions similar to a pacemaker, generating an electrical stimulus synchronously with breathing (Kezirian et al., 2010; Maurer et al., 2012). Several studies have shown that electrical stimulation in the hypoglossal nerve through this device improves the airspace of the oropharynx and hypopharynx, thus decreasing obstructive episodes during sleep by up to 68% (Kezirian et al., 2010; Lorenzi-Filho et al., 2017; Maurer et al., 2012). However, the implantation of this device is done in an invasive way and is highly irreversible.
Furthermore, there is a new therapeutic horizon that until now has not been explored in OSA: the use of sensory recovery interventions based on the concepts of neuroplasticity that are the basis of neurological rehabilitation (Gillick & Zirpel, 2012; Howlett et al., 2015; Page et al., 2015; Robbins et al., 2006). In fact, there is already preliminary information on the effectiveness of such interventions in the recovery of the sensitivity of patients with oropharyngeal dysphagia (Gallas et al., 2010; Pauloski et al., 2013; Rofes et al., 2013).
The evidence provided by previous research on the effectiveness of electrostimulation in OSA supports the potential of such interventions for the treatment of this entity, as long as stimulation is applied on the dilating muscles of the airway and stimulation of the constrictor muscles is avoided. Likewise, there is evidence that supports the potential benefit of electrostimulation in the recovery of sensory alterations of the laryngopharyngeal tract, which may have a role in the OSA pathogenesis. However, there are a number of problems with this treatment that have yet to be solved, including the development of a non-invasive, effective device; the alteration in sleep architecture by micro-arousals, or awakenings caused by the electric current; and the lack of a device for that a patient can used with minimal level training, on an outpatient basis.
How effective a new electrostimulation device is in dilating the upper airway, improving AHI, desaturation indices and quality of life in patients with obstructive sleep apnea
To evaluate in a first clinical trial a rehabilitation device for patients with OSA based on oropharyngeal electrical stimulation, which strengthens the dilating muscles of the upper airway, improves mechanical laryngopharyngeal sensitivity and improves OSA.
Perform experimental electrotherapy, nasoendoscopy, polygraphy and polysomnography tests with the device in order to calculate thresholds of functional and sensory intensities on the dilating muscles of the upper airway in patients with OSA. These tests also include the detection of signals that measure breathing events during sleep in patients with OSA in order to use them in the control mechanisms of the electrostimulation device.
Assess the effects and safety of the device in a group of five healthy volunteers for preliminary results.
Evaluate in a small group of patients with OSA the device capacity to stimulate the upper-airway dilating muscles and to reduce the AHI and oxygen desaturation indices when the electrostimulation device is used over a medium-term period (eight weeks) with morning and evening therapy sessions.
A feasibility clinical study of a medical device (Abdel-Aleem, 2009; CDHR et al., 2013) will be performed in patients prospectively recruited at the Sleep Medicine Service of Fundacion Neumologica Colombiana a tertiary care referral university medical centre specialized in respiratory and sleep medicine, located at Bogota. Patients will initially be evaluated by the Pulmonary and Sleep Medicine Service of Fundacion Neumologica Colombiana to confirm the diagnosis of OSA, for which the presence of symptoms of the disease plus a basal overnight polysomnogram with an AHI greater than 5 will be required.
In total, 12 patients with OSA will be recruited from those referred for full overnight polysomnography: four with mild, four with moderate and four with severe OSA. Patients will be recruited prospectively and consecutively after explaining the study and signing the informed consent. They will be evaluated by a pulmonary and sleep medicine doctor to confirm the diagnosis of OSA. For diagnosing OSA it will be required the presence of symptoms of the disease plus a baseline polysomnogram with an apnea-hypopnea index (AHI) greater than 5/h. Their general medical condition will also be evaluated and their admission to the protocol defined in accordance with the inclusion / exclusion criteria. Afterwards, the patients will undergo a clinical evaluation by a speech language pathologist trained in myofunctional therapy for OSA (see Figure 1). The study will also include a group of 5 healthy volunteers. The standard clinical evaluation of all patients and healthy volunteers will include the application of a standardized questionnaire assessing for the comorbidities of the Charlson Comorbidity Index (Charlson et al., 1994). Candidates for participating as healthy volunteers will also be screened with the STOP-BANG questionnaire (Baldini et al., 2017; Chung et al., 2016). A healthy volunteer will be define by the absence of signs and symptoms of acute conditions and comorbidities, and a STOP-BANG score < 3 (Baldini et al., 2017; Chung et al., 2016).
The classifications of the subjects in each category of OSA and as healthy controls will be done by pulmonary and sleep medicine physicians after the standard clinical evaluation. If it is considered that the subject applies as a control, they will be included in the study after signing the informed consent The definition of mild, moderate or severe OSA will depend on the presence of symptoms plus an AHI (measured during a full overnight polysomnography) between 5 and 15 in the case of mild OSA, >15 to <30 in the case of moderate OSA and ≥ 30 in the case of severe OSA.
Sample size calculation. Considering that this will be the first and pilot study of the electrostimulation device, there are no previous studies to make precise sample size calculations. Therefore, we considered it more appropriate to calculate the sample size using the effect size, that is, how large is the difference in the AHI before and after the intervention in terms of the standard deviation (SD) of the AHI (Cohen, 1988; Machin et al., 2009; Rosenthal, 1996). This method avoids the requirement of knowing the precise values for the means and SDs of the variables under study. For this pilot study, in which the priority is assessing feasibility, we considered that the AHI difference before and after the intervention should correspond to a large effect size to be considered significant. Future studies looking for smaller differences will use the results of this pilot study for sample size calculations. Effect size larger than 0.8 are considered large (Cohen, 1988; Rosenthal, 1996).
Therefore, the sample size was calculated looking for an effect size of 1.0 (a decrease in the AHI after the intervention of 1.0 SD of the AHI, which could correspond to about 15 apnoea-hypopnoea events per hour (Butler et al., 2019)), assuming a significance of 95%, a power of 80% for comparing repeated measures (Machin et al., 2009). With such assumptions, we calculated a minimum sample size of 10 subjects to detect statistically significant differences in the repeated measures comparison of pre-and post-intervention AHI (Machin et al., 2009), and two subjects were added to compensate for the potential loss to follow-up, for a total of 12 subjects.
Inclusion criteria for patients. Patients over 18 years of age with confirmed OSA (four with mild OSA, four with moderate OSA and four with severe OSA) who sign their informed consent to participate in the study.
Inclusion criteria for controls:
Exclusion criteria for patients and controls:
1. Pregnancy
2. Basal polysomnography that does not meet validity criteria to be interpreted.
3. Anticoagulation (although not a contraindication for laryngopharyngeal sensory endoscopic testing, anticoagulation is an exclusion criterion for this study to maintain the lowest level of risk).
4. Haemorrhagic diathesis (to avoid risk of severe epistaxis during nasal endoscopy).
5. Glasgow scale less than 15 (to avoid confusion with sensory or motor laryngopharyngeal involvement due to neurological disease that compromises the state of consciousness).
6. Basal oxygen saturation by awake pulse oximetry below 88%.
7. Patients with more than 5% of the total apnoea events being of central origin measured during a full overnight polysomnography(to avoid including patients with central sleep apnoea in whom laryngopharyngeal electrostimulation would have no effect).
8. Inflammatory or infectious lesions on the face or neck
9. Skin hypersensitivity
10. Anaesthetic areas, burns, bruises or recent wounds in the area of electrical stimulation
11. Cardiac pacemakers or other telemetry-controlled devices,
12. History of maxillofacial or pharyngeal surgery.
13. Active cancer
14. Tumours of the laryngopharyngeal tract.
15. Significant mental and/or behavioural conditions or inability of the patient to cooperate during the examination/intervention.
16. Epilepsy
17. Central nervous system (CNS) surgery in the last three months (to avoid confusion with muscle or sensory laryngopharyngeal compromise).
18. Brain trauma in the last three months (to avoid confusion with muscle or sensory laryngopharyngeal compromise).
19. Neurological sequelae of any cause compromising the head and neck muscles (to avoid confusion with muscle or sensory laryngopharyngeal compromise).
20. Underlying neuromuscular diseases affecting the head and neck muscles (to avoid confusion with muscle or sensory laryngopharyngeal involvement due to neuromuscular disease).
21. Chronic use of systemic corticosteroids at doses greater than or equal to 20 mg daily of prednisone or equivalent (to avoid confusion with corticosteroid myopathy that compromises the laryngopharyngeal region).
22. Bone prostheses or osteosynthesis (with polarized currents there is danger of chemical burn and bone resorption)
23. Acute febrile processes
24. Chronic decompensated diseases
25. Diseases in terminal states
26. Refusal to participate in the study.
Additional exclusion criteria for controls: The following exclusion criteria will apply to controls in order to ensure that they do not have significant comorbidities:
1. Previous diagnosis of OSA
2. STOP-BANG score >3 (Baldini et al., 2017; Chung et al., 2016)
3. Age > 60
4. Body mass index > 30
5. Any comorbidity of the Charlson Comorbidity index (Charlson et al., 1994) determined by the physician evaluating the selection criteria.
Anthropometric variables, including weight in kilograms, size in metres, body mass index in kilograms/squared meters (BMI), and the neck's circumference in centimetres, will be measured. In addition, the Epworth Sleepiness Scale (Johns, 1991), the Sleep Apnoea Quality of Life Index (SAQLI) (Coman et al., 2016) including the CPAP tolerance domain, will be applied for OSA patients and the STOP-BANG questionnaire for controls (Baldini et al., 2017; Chung et al., 2016). A full list of variables is provided in Table 1.
Candidates to be enrolled (patients and controls) will receive detailed information about the study and will sign their informed consent to participate in it.
Electrostimulation. Experimental tests with transcutaneous electrical stimulation at different intensities and at the points of the submandibular region previously explored by this and other research groups (Rangel-León et al., 2015) will be conducted first of all on the five healthy volunteer subjects. For the electrostimulation, the experimental device will be used. The stimulation with the electrode will be carried out on the upper airway dilator muscles, starting with the extralaryngeal muscles and then stimulating facial muscles. Electrostimulation will be performed in specific facial and extralaryngeal muscles, using a current type Biphasic Symmetric TENS, with a phase duration of 100 microseconds (µs), a frequency of 40 Hertz (Hz), an intensity of about 12 milliamps (mA) and a constant voltage, during a time of 15 minutes. The current intensity will be titrated to the tolerance of the patient or until obtaining visible muscle contraction. During stimulation, the region corresponding to the carotid sinus and the stellate ganglion will be avoided. During these tests, the neuromuscular functional and sensory thresholds of the upper airway, the most effective points of electrostimulation and the tolerance of intervention will be determined. The determination of the functional threshold will be made by clinical evaluation of the subject during stimulation (determining the current intensity required to induce contraction of the stimulated muscle).
Electrostimulation begins with electrodes placed on the skin in the submentonean and submandibular triangles (Figure 2), making sure not to place electrodes on the carotid sinus. The goal of this stimulation will be topographical closeness of the genioglossus, geniohyoid, mylohyoid, platysma and digastric muscles. For this purpose, symmetrical biphasic transcutaneous electrical nerve stimulation type (TENS-type) currents will be used, with a phase duration of 100 microseconds (µs), a frequency of 40 Hz and an intensity between 9–12 milliamps (mA), until the patient cannot tolerate electrostimulation or until visible muscle contraction (Rangel-León et al., 2015).
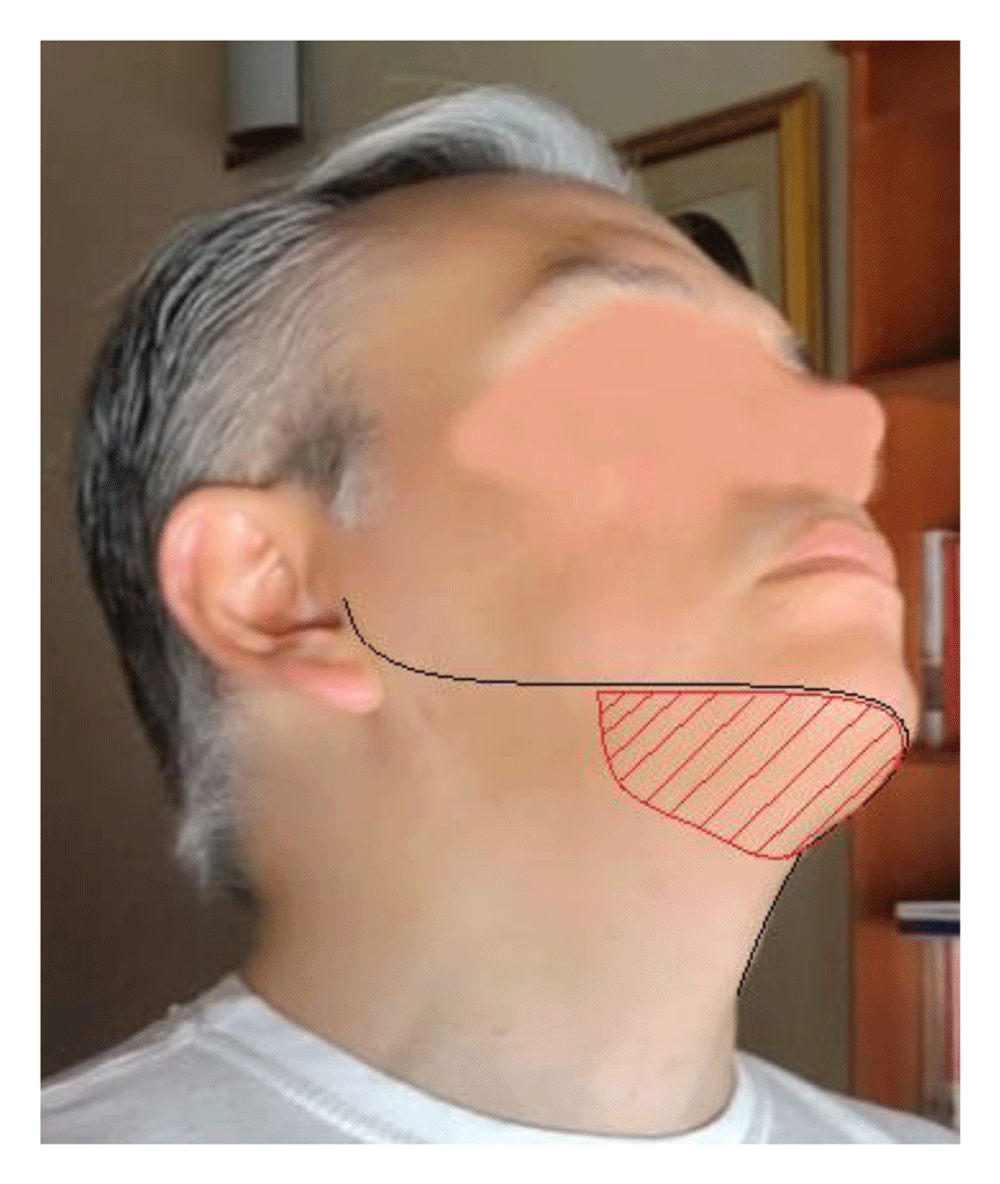
The electrodes for electrostimlation will be placed in the red area.
The functional threshold corresponds to the smallest current intensity capable of obtaining contraction of the airway's dilating muscles. The presence of contraction of the stimulated muscles will be determined by asking the patient about the sensation they feel, as well as by external and endoscopic inspection. These thresholds will be measured using the experimental device, which provides real-time information of the current characteristics (including intensity, voltage and frequency). The sensory threshold corresponds to 75% of the functional threshold’s current intensity (Robbins et al., 2006; Rofes et al., 2013). The degree of opening of the upper airway obtained on these thresholds will be determined by conventional video-flexible-nasolaryngoscopy measuring the upper airway diameters using software designed ad hoc and previously validated.
After characterizing the electrostimulation thresholds in healthy volunteers (functional and sensory thresholds), as well as their desired and secondary effects, the 12 subjects with OSA will undergo electrostimulation at the same area of the neck using similar current characteristics and titrating the current intensity to determine the functional and sensory thresholds for each patient. The neuromuscular thresholds of the upper airway and the most effective points of electrostimulation will be determined during these tests. Again, the determination of the functional threshold will be made by clinical evaluation of the subject during stimulation, and the degree of opening of the upper airway based on these thresholds will then be evaluated by conventional video-flexible-nasolaryngoscopy.
We will conduct an analysis of the correlation of the signals obtained in experimental tests, elaborate a map of more effective points for electrical stimulation and determine the intensities required for each patient. We will assess the possibility of using the same standard point map for all patients.
Afterwards, the 12 OSA patients will receive an eight-week therapeutic intervention, using a protocol similar to that used by our group (Rangel-León et al., 2015). The effects of the intervention will be evaluated looking for improvement of the AHI, the desaturation indices, the Epworth Sleepiness Scale (ESS) (Johns, 1991) and the Sleep Apnoea Quality of Life Index SAQLI. (Coman et al., 2016). The improvement of the AHI and desaturation indices will be determined by comparing full overnight polysomnograms conducted before and after the intervention and looking at the changes in such indices. Polysomnograms will include standard electroencephalography, electrooculography, mandible and leg electromyography, nasal and oral airflow, and electrocardiography; respiratory effort will be measured by diaphragmatic excursion, and oxygen saturation will be measured by pulse oximetry. Polysomnograms will be performed, read and interpreted in the sleep laboratory of the Colombian Pneumological Foundation by certified personnel blinded to the patient intervention. The person in charge of reading and interpreting the polysomnograms will be blinded to the patient’s intervention. Sleep stages and respiratory events (including apnoea-hypopnoea and desaturation events) will be scored according to the rules of the American Academy of Sleep Medicine (AASM) Manual for Scoring of Sleep and Associated Events (Berry et al., 2012). The clinical diagnosis of OSA will be defined by an apnoea-hypopnea index (AHI) > 5 events/hour of sleep, with symptoms of sleep fragmentation and daytime somnolence according to the American Academy of Sleep Medicine. The severity of OSA will be determined according to the AHI as follows: mild, 5–14.9; moderate, 15–30; and severe > 30 events/hour (Takegami et al., 2009).
Patients will undergo a conventional video-flexible-nasolaryngoscopy in which the degree of obstruction and the laryngopharyngeal sensitivity during wakefulness will be determined in accordance with the protocol previously validated by our research group (Giraldo-Cadavid et al., 2017; Giraldo-Cadavid et al., 2017). Briefly, the upper airway mucosa is stimulated at precise points (aryepiglottic folds, lateral pharyngeal walls and soft palate) using air-pulses of different intensities, the sensory thresholds correspond to the lower air-pulse intensity perceived by the patient (Giraldo-Cadavid et al., 2017; Giraldo-Cadavid et al., 2017). This test is performed using a thin flexible endoscope with a working channel of 1.2 mm. The endoscopist introduces the endoscope through the patient’s nasal cavity. It is lubricated with water-soluble gel to decrease discomfort. The endoscopist does not use anesthetics because the test only produces mild to moderate discomfort, it is infrequently associated with pain, and anesthetics might alter the laryngopharyngeal tract reflexes and the measurements of their thresholds.
The sensory evaluation consists of administering pulses of air at different intensities to the patient to stimulate and determine the reflex thresholds of the laryngeal adductor (LART), cough (CRT), gag reflexes (GRT) and psychophysical sensitivity thresholds.
Effectiveness assessment will also include video-flexible-nasolaryngoscopies with measurement of laryngopharyngeal sensitivity as previously detailed, and Drug-induced sleep endoscopy (DISE) each performed before the intervention and a second of each test performed after the intervention. During DISE the level and degree of upper airway obstruction will be determined at the soft palate, tonsils, tongue base and the epiglottis using software designed ad hoc for measuring the airway diameter and perimeter and previously validated.
The image processing software is made in Python and determines the airway obstruction degree as follows:
The system takes the video made during DISEs, divides it into image format frames for edition. Subsequently, the rater selects the frames with the airway region where he wants to make the measurements while there are not OSA events, usually in the initial phase of sedation (in light sleep), he also selects frames with the same airway regions to make the measurements during the OSA events, usually in more profound stages of sleep. In each frame, the rater delimits the areas and diameters to be measured in the image using the computer's mouse. The software processes the images to calculate the areas' and diameters' size in pixels and displays their corresponding values in a graph. To calculate the percentage of obstruction the computer divides the measures of the areas and diameters during OSA events by the corresponding measures taken at the beginning of the DISE (during the lightest stage of sleep) when there are not obstructive sleep apnoeas.
The pre-intervention tests, including clinical evaluation (history, anthropometric measures and physical exam), basal polysomnogram, SAQLI, ESS, STOP-BANG, and DISE will be performed a maximum of one month before the start of the intervention, and the post-intervention tests will be performed a maximum of one month after the intervention.
During the study, patients will continue with the therapy ordered by their treating physician for the management of OSA, including CPAP and lifestyle changes.
Any adverse event will be notified to the institutional review board and competent authorities in the expected time according to its severity: less than 24 hours in case of serious adverse events.
The effectiveness assessment will be based on the improvement of the following outcomes:
AHI during sleep.
AHI during rapid eye movements (REM) sleep.
AHI during non-REM sleep.
Desaturation index: number of desaturations per hour during sleep.
T90: percentage of sleep time spent under 90% oxygen saturation.
SpO2 nadir: lowest oxygen saturation measured by pulse oximetry.
Mean SpO2: mean oxygen saturation measured by pulse oximetry.
Number of patients who lower one or more categories in the severity of OSA (moving from severe to moderate, severe to mild or moderate to mild OSA or normalising the AHI).
Number of patients who climb one or more categories in the severity of OSA (moving from mild to moderate, mild to severe or moderate to severe OSA).
Improvement in the quality of life as measured by SAQLI.
Improvement in daytime sleepiness as measured by the ESS.
Qualitative variables will be described with absolute and relative frequencies, and comparisons between before and after the intervention will be made by the McNemar test.
Quantitative variables will be evaluated for assumptions of normality by the Shapiro-Wilk test. If they have normal distribution, they will be described with means and standard deviations; otherwise, they will be described with medians and interquartile ranges. Comparisons of before and after the intervention will be made by student t-tests for paired measurements if the distribution is normal or by the Wilcoxon signed range tests otherwise.
Statistical analysis will be performed using Stata version 16.1 (StataCorp, Lakeway Drive, USA).
The type of electric current that will be used in this study is safe in its application with a low probability of causing burns or adverse events in deep tissues. Electrostimulation is an invention widely used today for pain management for cancer, precordial pain, herpes, vertebral pain and immediate postoperative pain (Rodríguez, 2004). However, because new and experimental equipment not yet approved by regulatory bodies will be used, there is a higher than minimum risk present. This protocol was approved by the ethics committee (IRB) of Fundación Neumológica Colombiana with act number 234 of March 2, 2018 any modification in this protocol will be submitted to the IRB for approval before its implementation.
The study will be explained to each patient verbally and in a written document that summarises the objectives and characteristics of the study. Informed written consent will be obtained from patients who agree to enter the study.
The database with patient information will be anonymised to preserve patient confidentiality. Insurance will be taken out to cover the risks of side effects in patients.
This protocol was registered with ClinicalTrials.gov on 29th October 2020 (NCT04607343).
The study results will be summarized and presented in at least one international and one national medical conference. Afterwards a final report in the form of a scientific original article will be submitted to an international peer review journal.
Not yet recruiting.
Open Science Framework: Clinical trial of a rehabilitation device based on electrical stimulation for patients with obstructive sleep apnoea (OSA): a study protocol. https://doi.org/10.17605/OSF.IO/AU8F4 (Giraldo-Cadavid et al., 2021)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: Inspire, LivaNova, Nyxoah, Medel: research regarding different OSA treatments and physiology of OSA
Reviewer Expertise: Sleep medicine, functional treatment, obstructive sleep apnoea
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Not applicable
References
1. O'Connor-Reina C, Rodriguez-Alcala L, Ignacio JM, Baptista P, et al.: Assessment of Muscular Weakness in Severe Sleep Apnea Patients: A Prospective Study.Otolaryngol Head Neck Surg. 2023; 169 (3): 725-733 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: sleep disordered breathig
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Mar 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)