Keywords
Psilocybin, Psilocin, Magic mushroom, Gold cap mushroom, PBPK model
Psilocybin, Psilocin, Magic mushroom, Gold cap mushroom, PBPK model
Magic mushroom (Psilocybe cubensis), also called gold cap mushroom, can be found in tropical and subtropical areas around the world. Magic mushroom is a psychedelic mushroom that can cause euphoria, hallucinations (mental, auditory) and changes in perception1. Psilocybin (PB) and psilocin (PI) are the major psychoactive compounds in the mushrooms2.
PB (4-phosphoryloxy-N,N-dimethyltryptamine) is a compound found in the mushroom in a psychologically inactive form. It can be orally absorbed (F = 0.5) following single oral administration in humans2. After being orally absorbed, PB is rapidly dephosphorylated by alkaline phosphatase and non-specific esterase to produce an active metabolite, namely PI (4-hydroxy-N,N-dimethyltrypt-amine)2,3. Since PI is structurally similar to serotonin, PI can bind and activate several receptors in the brain such as 5-HT2A, 5-HT1A, 5-HT1D, and 5-HT2C receptors3. Activation through the 5-HT2A receptor can lead to the psychedelic effects of the mushrooms4. PI can be glucoronidated by UDP-glucuronosyltransferase (UGT) 1A10 in the liver and intestinal mucosa5.
There have been studies of PB for treatment of some psychological disorders such as depression, suicidal attempts, obsessive-compulsive disorder (OCD), alcohol use disorder, tobacco use disorder and resistant depression5,6. However, knowledge of how PB and PI behave in the body is still incomplete, particularly information concerning PI concentration levels in the brain, the major target organ of the magic mushroom. As a result, dosing of PB and PI in humans can lead to inadequate outcomes. Physiologically-based pharmacokinetic (PBPK) modeling is a quantitative tool capable of predicting concentration-time profiles of chemicals including drugs, toxicants and natural products7–12. Any biologically relevant process related to the PK of the drugs can be incorporated into the model. To be able to quantify brain concentration levels of PI in human brains following PB administration, a PBPK model of PB and PI with a description of a brain compartment can be useful in understanding the disposition of PI. Therefore, the objective of this study was to develop a PBPK model of PB and PI in rats and humans.
PBPK model structure. A PBPK model structure consists of seven compartments including the lung, heart, brain, fat, muscle, kidney, and liver. All of the organ compartments are linked together by the blood circulation system. The lung is the site of an interchange of the arterial and venous blood. The brain is the major site of action of PI. PI is assumed to solely be eliminated from the body through the liver. Adipose tissue is the storage organ for PI in the body. Additional organs including the heart and kidney are included in the model regarding their role in the pharmacokinetics and pharmacodynamics of PI. The structure of our PBPK model of PB and PI is depicted in Figure 1.
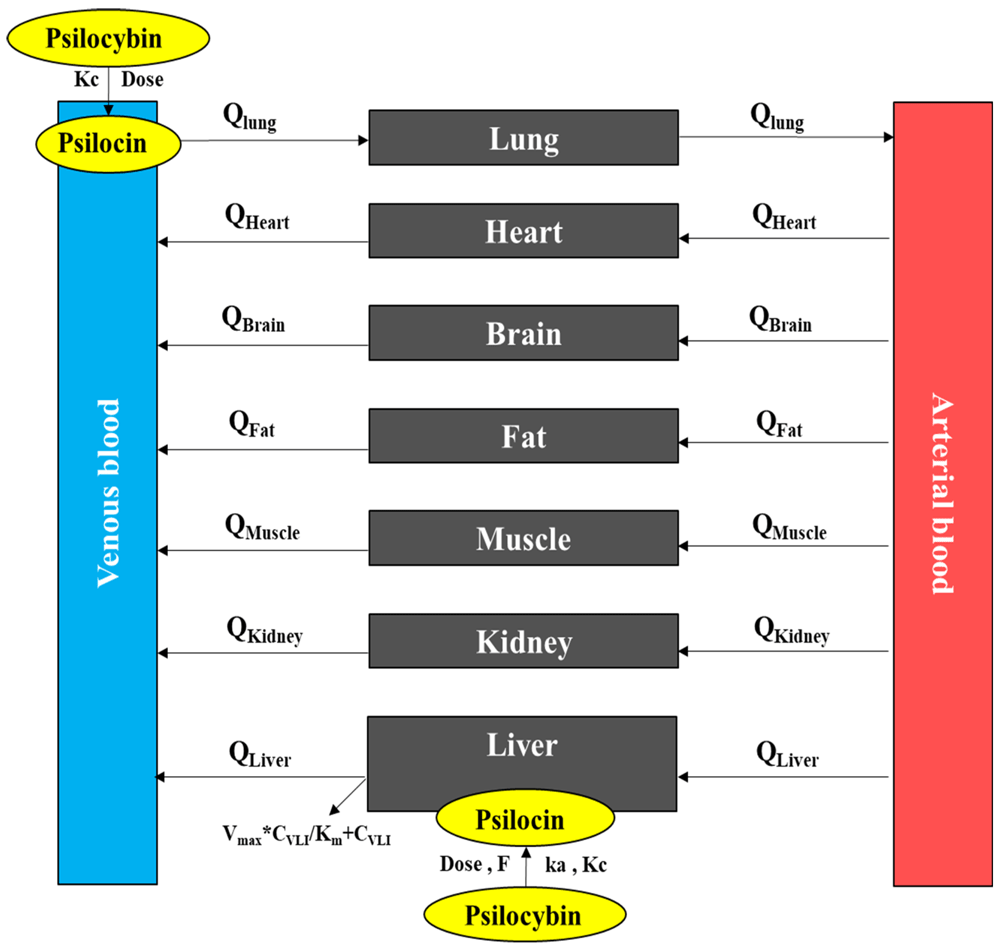
Tissue model specification. A perfusion rate-limited and well-stirred tissue model hypothesis was assumed for all organs.
Model parameterization and equations. Physiological parameters including organ blood flow (Q) and organ volume (V) were obtained from published literature13–15. Pharmacokinetic parameters including bioavailability and absorption rate constant were acquired from published literature16,17 and optimized using Berkeley Madonna Software. Physicochemical parameters including partition coefficient of each compartment were calculated using an approach from the literature18. A PBPK model was coded and performed using Berkeley Madonna Software version 8.3.18 (developed by Robert Macey and George Oster of the University of California at Berkeley). Replication of the simulation results from our PBPK model using an alternative and freely available software is possible; recently, Zurlinden T.J. et al.19 have developed a physiologically based pharmacokinetic model of a rifapentine model using the Python software (version 2.7.2) as their computational tool along with these numby, scipy and matplotlib packages20–23. All of the parameters used in the PBPK model are summarized in Table 1.
| Parameters | Rats | Reference | Humans | Reference |
|---|---|---|---|---|
| Blood flow fraction | ||||
| Fat (QFC) | 0.07 | 13 | 0.021 | 14 |
| Liver (QLIC) | 0.183 | 13 | 0.1195 | 14 |
| Muscle (QMUC) | 0.278 | 13 | 0.0606 | 14 |
| Kidney (QKC) | 0.141 | 13 | 0.1001 | 14 |
| Brain (QBRC) | 0.02 | 13 | 0.0565 | 14 |
| Heart (QHC) | 0.051 | 13 | 0.0194 | 14 |
| Tissue volume fraction | ||||
| Fat (VFC) | 0.076 | 15 | 0.1429 | 14 |
| Liver (VLIC) | 0.0366 | 15 | 0.0241 | 14 |
| Muscle (VMUC) | 0.404 | 15 | 0.5 | 14 |
| Lung (VLUC) | 0.005 | 15 | 0.0167 | 14 |
| Kidney (VKC) | 0.0073 | 15 | 0.004 | 14 |
| Brain (VBRC) | 0.0057 | 15 | 0.0207 | 14 |
| Heart (VHC) | 0.0033 | 15 | 0.0044 | 14 |
| Vein (VVC) | 0.0544 | 15 | 0.0496 | 14 |
| Artery (VAC) | 0.0272 | 15 | 0.0247 | 14 |
| Partition coefficients | ||||
| Fat (PF) | 0.1484 | 18 | 0.1818 | 18 |
| Liver (PLI) | 1.0749 | 18 | 1.5383 | 18 |
| Muscle (PMU) | 0.8781 | 18 | 1.1732 | 18 |
| Lung (PMU) | 1.2035 | 18 | 0.7665 | 18 |
| Kidney (PK) | 1.0799 | 18 | 1.1873 | 18 |
| Brain (PMU) | 1.8662 | 18 | 2.1357 | 18 |
| Heart (PH) | 1.0046 | 18 | 0.9673 | 18 |
| Molecular weight (g/mole) | 204.27 (PI) | 24 | 284.25 (PB) | 25 |
| Maximum velocity (Vmax), μmole/h | 270* | Optimized | 1,450-2,080* | Optimized |
| Michaelis constant (Km), μM | 1,080* | Optimized | 50* | Optimized |
| Bioavailability (F) | 0.52* | Optimized | 0.52717-0.9* | Optimized |
| Absorption rate constant (ka), 1/h | 0.16* | Optimized | 0.36716-0.67* | Optimized |
| Conversion rate constant (KC) | - | Optimized | 0.4* | Optimized |
Routes of PI administration. In this development of the PBPK model of PB and PI in rats and humans, there were two different routes of administration. Those included 1) intravenous (IV) administration and 2) oral administration. Differential equations (Equation 1 – Equation 3) describing the respective routes of administration are as follows:
1) Intravenous administration:
where Dose is an administered dose of PI (mg), BW is the body weight (kg), Time is the duration period of the injection (h), and Kc is the conversion rate constant.
2) Oral administration:
where AGU is the amount of PI in the gut lumen after an oral administration (μg), ka is the absorption rate constant (1/h), F is the bioavailability, and Kc is the conversion rate constant.
Organ compartments. In this PBPK model, there were seven organ compartments. Differential equations (Equation 4 – Equation 20) describing concentration-time profiles of PI in each of seven compartments are as follows:
1) Lung:
where ALU is the amount of PI in the lung (µg), QC is the cardiac output (l/h), CV is the concentration of PI in venous blood (µg/l), CALU is the concentration of PI in arterial blood leaving the lung (µg/l), CLU is the concentration of PI in the lung (µg/l), VLU is the volume of the lung (l), and PLU is the lung/blood partition coefficient.
2) Heart:
where AH is the amount of PI in the heart (µg), QH is the blood flow to the heart (l/h), CA is the concentration of PI in arterial blood (µg/l), CVH is the concentration of PI in venous blood leaving from the heart (µg/l), CH is the concentration of PI in the heart (µg/l), VH is the volume of the heart (l), and PH is the heart/blood partition coefficient.
3) Brain:
where ABR is the amount of PI in the brain (µg), QBR is the blood flow to the brain (l/h), CA is the concentration of PI in arterial blood (µg/l), CVBR is the concentration of PI in venous blood leaving from the brain (µg/l), CBR is the concentration of PI in the brain (µg/l), VBR is the volume of the brain (l), and PBR is the brain/blood partition coefficient.
4) Fat:
where AF is the amount of PI in the fat (µg), QF is the blood flow to the fat (l/h), CA is the concentration of PI in arterial blood (µg/l), CVF is the concentration of PI in venous blood leaving from the fat (µg/l), CF is the concentration of PI in the fat (µg/l), VF is the volume of the fat (l), and PF is the fat/blood partition coefficient.
5) Muscle:
where AMU is the amount of PI in the muscle (µg), QMU is the blood flow to the muscle (l/h), CA is the concentration of PI in arterial blood (µg/l), CVMU is the concentration of PI in venous blood leaving from the muscle (µg/l), CMU is the concentration of PI in the muscle (µg/l), VMU is the volume of the muscle (l), and PMU is the muscle/blood partition coefficient.
6) Kidney:
where AK is the amount of PI in the kidney (µg), QK is the blood flow to the kidney (l/h), CA is the concentration of PI in arterial blood (µg/l), CVK is the concentration of PI in venous blood leaving from the kidney (µg/l), CK is the concentration of PI in the kidney (µg/l), VK is the volume of the kidney (l), and PK is the kidney/blood partition coefficient.
7) Liver:
where ALI is the amount of PI in the liver (µg), QLI is the blood flow to the liver (l/h), CA is the concentration of PI in arterial blood (µg/l), CVLI is the concentration of PI in venous blood leaving from the liver (µg/l), CLI is the concentration of PI in the liver (µg/l), VLI is the volume of the liver (L), PLI is the liver/blood partition coefficient, LIMet is the amount of PI excreted via liver metabolism, Vmax is the maximum velocity of UGT1A10 (μmol/h), and Km is Michaelis-Menten constant of UGT1A10 (μM).
8) Blood:
where AV and AA are the amount of PI in the venous and arterial blood, QH, QBR, QF, QMU, QK, QLI are the blood flow rate into the heart, brain, fat, muscle, kidney, and liver (l/h), and CVH, CVBR, CVF, CVMU, CVK, CVLI are the concentration of PI in the venous blood leaving from the heart, brain, fat, muscle, kidney, and liver (μg/).
From a search in PubMed from inception to July 2019, the search terms “psilocybin; psilocin; magic mushroom; pharmacokinetic” were used as keywords. Studies were included if they met the selection criteria as follows: pharmacokinetic studies of psilocybin or psilocin that were conducted in animals or humans and different of routes of administration. Studies were excluded if they conducted pharmacokinetic studies in unhealthy or pregnancy animals or humans. From the following information in the literature, three studies were retrieved and were subsequently used for further model development. The PBPK model was evaluated based on visual inspection of goodness of fit. A summary of the selected studies is as follows:
In the study by Chen et al.26, Sprague-Dawley rats (n = 10, BW = 220 – 250 g) were orally administered with a single dose of G. spectabilis extract, which is equivalent to 10.1 mg/kg of PI. Then, a serial of blood samples was collected at 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, 360, and 420 minutes after oral administration. Then, the samples were analysed for PI using ultra-performance liquid chromatography coupled to photodiode array detection (UPLC-PDA).
In the study by Hasler et al.17, nine healthy male volunteers (BW = 56 – 72 kg) were intravenously administered with a single dose of 1 mg PB. Subsequently, blood samples were collected at 0.75, 1.5, 2.5, 3.75, 5, 6.75, 10, 15, 20, 30, 60, and 120 minutes. In addition, six healthy male and female volunteers (BW = 53 – 88 kg) were orally administered with a single oral dose of 0.224 mg/kg PB. Blood samples were collected at 15, 30, 4.5, 60, 7.5, 90, 105, 120, 150, 180, 220, 300, 350, and 390 min after the oral PB administration. Then, the samples were analysed using high performance liquid chromatography coupled with electrochemical detection (HPLC-ECD).
In Brown et al.16, 12 healthy volunteers were given a single oral administration of PB 0.3 mg/kg. Blood collections were performed at 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 12, 18, and 24 hours. Subsequently, the samples were analysed for PI concentration using a HPLC technique.
PI concentration levels shown in figures for the selected studies were extracted using WebPlotDigitizer version 4.4 (Free Software Foundation, Inc., Boston, MA). Assessment of model qualification was based on visual inspection of the agreement between observed and simulated PI concentrations. Regression analysis between the simulated and observed PI levels was also performed in Microsoft Excel version 2019.
Following a single oral administration of PI (10.1 mg/kg) in rats, the simulated results of PI concentration profiles in plasma from the developed model compared to observed data acquired from the study by Chen et al.26 is demonstrated in Figure 2A with the simulated PI concentration-time profiles in the brain27. Subsequently, simulated results of PI concentration-time profiles in the lung, heart, muscle, kidney, and liver are illustrated in Figure 2B.
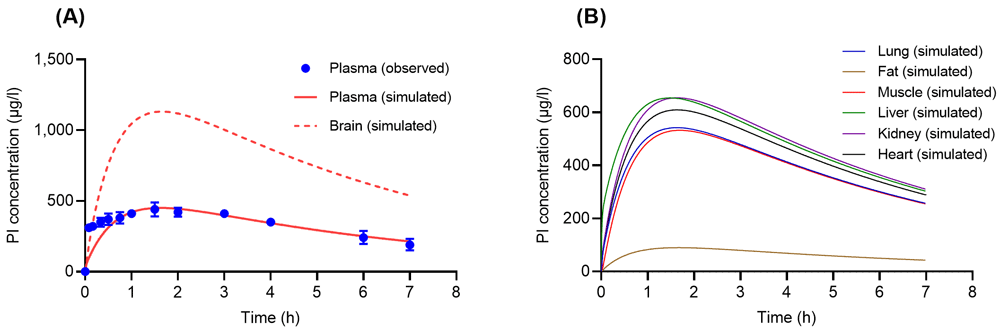
(A) Concentration-time profiles of psilocin (PI) in rat plasma and brain following a single oral administration of psilocin (PI) (10.1 mg/kg). Solid and dashed lines are concentration-time profiles of PI from the developed physiologically based pharmacokinetic (PBPK) model, whereas closed circles with error bars (S.E.) are experimental data acquired from Chen et al.26. (B) Simulated concentration-time profiles of PI following a single oral dose of PI (10.1 mg/kg) in rat’s tissues such as lung (blue solid line), fat (brown solid line), muscle (red solid line), liver (green solid line), kidney (purple solid line), and heart (black solid line).
Following a single IV administration of PB (1 mg) in humans, simulated PI concentration-time profiles in plasma compared to the observed data acquired from Hasler et al.17 are demonstrated in Figure 3A. In addition, PI concentration-time profiles in the human brain were simulated and are presented in Figure 3A. Simulated PI concentration-time profiles of other tissues are illustrated in Figure 3B.
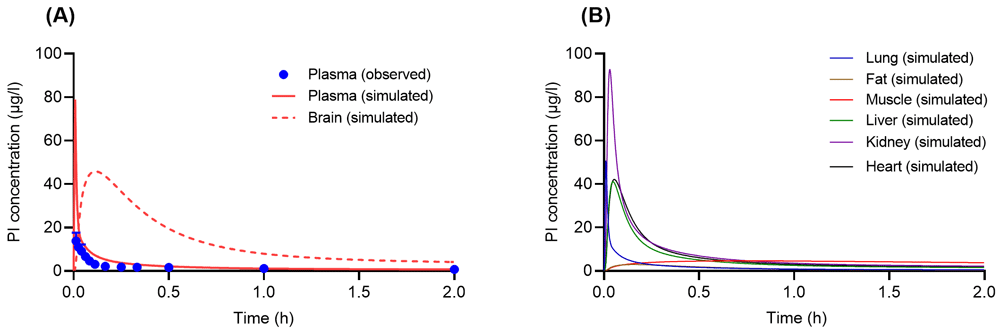
(A) Concentration-time profiles of psilocin (PI) in human plasma and brain following a single intravenous administration of psilocybin (PB) (1 mg). Solid and dashed line are concentration-time profiles of PI from the developed physiologically based pharmacokinetic (PBPK) model, whereas closed circles with error bars (S.E.) are experimental data acquired from Hasler et al.17. (B) Simulated concentration-time profiles of PI following a single intravenous dose of PB (1 mg) in human tissues such as lung (blue solid line), fat (brown solid line), muscle (red solid line), liver (green solid line), kidney (purple solid line), and heart (black solid line).
In the selected studies, two different doses of PB (0.224 mg/kg and 0.3 mg/kg) were administered to humans via a single oral administration. Simulated results of PI concentration-time profiles in plasma following oral administration of PB (0.224 mg/kg and 0.3 mg/kg) compared to the observed data acquired from Hasler et al.17 and Brown et al.16 are presented in Figure 4A and Figure 5A, respectively. Simulated brain concentration-time profiles of PI for both doses (0.224 mg/kg and 0.3 mg/kg) are also presented in Figure 4A and Figure 5A, respectively. Then, from the developed model following oral administration of PB (0.224 mg/kg and 0.3 mg/kg), the simulated results of PI in organs of interest including the lungs, fat tissue, muscle, kidney, and liver are depicted in Figure 4B and Figure 5B.
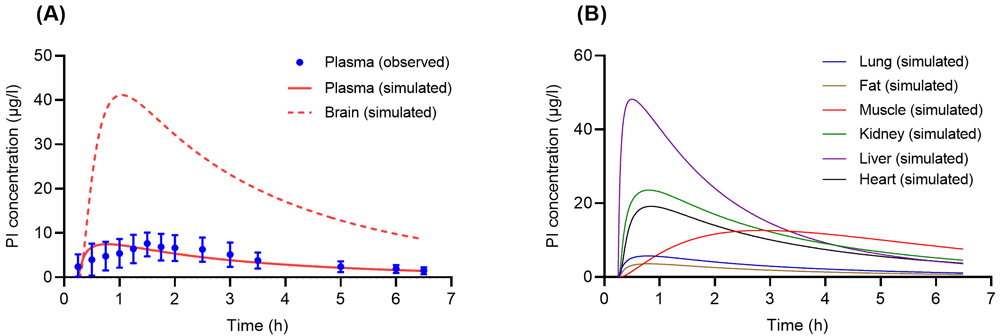
(A) Concentration-time profiles of psilocin (PI) in human plasma and brain following a single oral administration of psilocybin (PB) (0.224 mg/kg). Solid and dashed lines are concentration-time profiles of PI from the developed physiologically based pharmacokinetic (PBPK) model, whereas closed circles with error bars (S.E.) are experimental data acquired from Hasler et al.17. (B) Simulated concentration-time profiles of PI following a single oral dose of PB (0.224 mg/kg) in human tissues such as lung (blue solid line), fat (brown solid line), muscle (red solid line), liver (green solid line), kidney (purple solid line), and heart (black solid line).
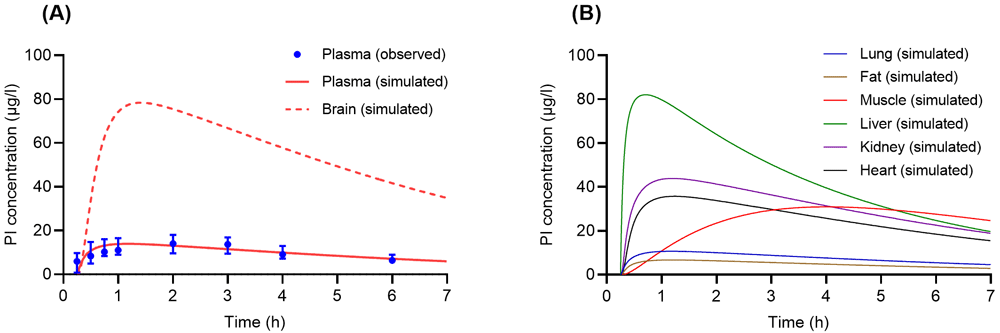
(A) Concentration-time profiles of psilocin (PI) in human plasma and brain following a single oral administration of psilocybin (PB) (0.3 mg/kg). Solid and dashed lines are concentration-time profiles of PI from the developed physiologically based pharmacokinetic (PBPK) model, whereas closed circles with error bars (S.E.) are experimental data acquired from Brown et al.16. (B) Simulated concentration-time profiles of PI following a single oral dose of PB (0.3 mg/kg) in human tissues such as lung (blue solid line), fat (brown solid line), muscle (red solid line), liver (green solid line), kidney (purple solid line), and heart (black solid line).
Our model simulations in both species included different routes of administration including an oral dose of PI in rats (10.1 mg/kg), IV dose of PB in humans (1 mg), and different oral doses of PB in humans (0.224 mg/kg and 0.3 mg/kg). Key parameters including maximum concentration (Cmax), time to reach maximum concentration (Tmax), and area under the curve from time equals zero to the last time point (AUC0-t) from the developed PBPK model and experimental data in both rats and humans are demonstrated in Table 2.
| Species | Study | Route of administration | Dose | Observed Cmax (μg/l) | Simulated Cmax (μg/l) | Observed Tmax (h) | Simulated Tmax (h) | Observed AUC0-t (μg.h/l) | Simulated AUC0-t (μg.h/l) | %AUC0-t deviation | R-square |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rats | Chen et al.26 | Oral | 10.1 mg/kg (PI) | 430 | 450.7 | 1.5 | 1.625 | 2,375 | 2,342 | 1.38 | 0.6 |
| Humans | Hasler et al.17 | IV | 1 mg (PB) | 12.9 | 78.46 | 0.031 | 0.008 | 4 | 5 | 25 | 0.8 |
| Oral | 0.224 mg/kg (PB) | 8.2 | 7.5 | 1.75 | 0.78 | 32.71 | 24.2 | 26 | 0.57 | ||
| Brown et al.16 | Oral | 0.3 mg/kg (PB) | 16 | 13.95 | 2.03 | 1.17 | 140 | 93.81 | 33 | 0.76 |
To our knowledge, this current PBPK model of PB and PI is the first model with the capacity to describe PI concentration-time profiles following an oral administration of PI in rats as well as an intravenous and oral administration of PB in humans. The developed PBPK model could describe PI concentration-time profiles in plasma from both species (Figure 2A, Figure 3A, Figure 4A and Figure 5A). Furthermore, the developed PBPK model could simulate PI concentration-time profiles in other tissues, particularly in the brain, which is the major target organ of the psychoactive compound in magic mushrooms.
However, under-predicting of blood PI levels at early time points after PB oral administration was observed (Figure 4A and Figure 5A). These under-predictions could be a result of the conversion rate constants used in the current PBPK model of PB and PI. In this model, the conversion rate constant was assumed as a constant in a zero-ordered kinetic process. However, the enzymatic reaction by the esterase enzyme system in the blood could be either a first-ordered kinetic process or Michaelis-Menten process. Nonetheless, our model outputs after two hours post-administration could capture most of the data points acquired from the selected studies reasonably well (Figure 2A, Figure 4A, and Figure 5A).
Following IV administration in human subjects, from time equals zero to about half an hour, model over-predictions were observed (Figure 3A). These over-predictions might be a result of 1) over-simplification from the well-stirred model hypothesis and 2) at the beginning of the IV administration, the administrated amount of PB could saturate the available enzymatic process for changing PB to PI in the body.
Our developed PBPK model of PB and PI is capable of making predictions of PI concentration-time profiles in the brain of both rats and humans. However, data concerning brain PI concentration-time profiles are lacking. From our literature search, there was a study by Law et al.28. In this study, pregnant rats were intravenously administrated with PI. Subsequently, tissue samples (i.e. brain, lung, fat, muscle, kidney, liver, spleen, placenta, and fetus) were harvested at different time points and PI concentration levels in the tissue samples were analyzed. However, this study was conducted in pregnant rats which, due to potential changes as a result of the physiology of the pregnancy, may significantly influence the pharmacokinetics of PB and PI. Consequently, we could not use this dataset in the current model development. Thus, to have more confidence in the developed PBPK model, further PI concentration-time profiles in tissue organs including the brain are necessary. Therefore, a new study in rats intravenously and orally administered with PB with an additional design of tissue harvesting (e.g. brain, fat, muscle, kidney, and liver) is warranted. In addition, to further extend the model’s application and to make an adequate prediction between the administered dose of PB and PI and its psychoactive effects, a physiologically based pharmacokinetic/pharmacodynamic (PBPK/PD) model for PB and PI in humans should be developed.
A PBPK model of PB and PI in rats and humans was developed. The PBPK model could predict concentration-time profiles of PI following intravenous and oral administration of PB. In addition, concentration-time profiles of PI in the brain, the major target organ of PI, could be simulated. The PBPK model of PB and PI may be useful for a safer dosage regimen for patients with some disorders for which PB or magic mushrooms may be therapeutically effective.
Zenodo: Heang60/PBPK-of-Magic-Mushroom: Coding for PBPK of Magic Mushroom. https://doi.org/10.5281/zenodo.453661727.
This project contains the following underlying data:
Zenodo: Heang60/PBPK-of-Magic-Mushroom: Coding for PBPK of Magic Mushroom. https://doi.org/10.5281/zenodo.453661727.
This project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental health, Environmental modeling, PBPK modeling, Air pollution
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Kalberer F, Kreis W, Rutschmann J: The fate of psilocin in the rat. Biochemical Pharmacology. 1962; 11 (4-5): 261-269 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacokinetics and PBPK modeling.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Mar 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)