Keywords
Organochlorine pesticides, prostate cancer, biochemical recurrence, advanced disease
Organochlorine pesticides, prostate cancer, biochemical recurrence, advanced disease
AO: Agent Orange; BCR: Biochemical Recurrence; OCs: Organochlorines; OCPs: Organochlorine Pesticides; PCa: Prostate Cancer
Prostate cancer (PCa) is the second most common non-cutaneous malignancy diagnosed among men worldwide, and the most common cancer type detected in men in developed countries1. Several risk factors for the development of PCa have been established, including increasing age, positive family history, and accumulated environmental exposure to several hormones2,3. Some pesticides can influence the hormonal milieu in vivo by functioning to mimic the effect of hormones, regulate enzyme systems involved in hormone metabolism, and affect androgenic stimulation of the prostate gland, potentially leading to increased cellular proliferation and progression to malignancy4–6. Organochlorines (OCs) comprise a large number of pesticides, and these have been used extensively throughout the world for several decades. Whilst their use has been banned or severely restricted in many countries, they remain in use in many areas of the world, and this has the potential to adversely affect the health of individuals in countries where OCs are still in use. OCs are highly-persistent organic pollutants, with a high serum level being reported in several distinct populations7–10. The International Agency of Research on Cancer (IARC) has classified many OCPs as being Class 2B agents, implicating them as being possible carcinogens11. Moreover, a large number of OCPs have been demonstrated to have the potential to disrupt endocrine function12,13, suggesting that exposure to these specific types of pesticides may increase the risk of developing hormone-dependant cancers such as PCa14. Several OCPs including chlordecone, DDE, DDT and Lindane have been implicated as potential independent risk factors for PCa development15–18. However, to date there is a relative lack of information about the impact of exposure to OCPs upon on the development of aggressive metastatic PCa, or influences on PCa disease-free survival, and potential BCR following radical treatment. The aim of this review article is to provide a contemporary update of the epidemiologic evidence implicating exposure to OCPs upon the recurrence of PCa following radical therapy.
We conducted a systematic review of the available epidemiological data investigating a potential relationship between exposure to OCPs and the development of recurrent PCa following radical therapy. We searched all peer-reviewed articles published up to July 31st 2020. Pre-defined eligibility criteria for the inclusion of studies were that they be original studies, reviews, previous meta-analyses, or case–control or cohort studies. Moreover, it was mandatory that they contain information about association measures, including odds ratios (OR), relative risks (RR), and confidence intervals (CI) in order to facilitate an analysis of possible relationships between exposure to specific OCPs and development of recurrent PCa following treatment. Finally, it was necessary for the included studies to provide sufficient data and be written in English, French, or Spanish. Exclusion criteria included in vitro experimental and mechanistic studies, editorials, or letters, and as such these reports were not included in this review.
The initial search strategy included PubMed MEDLINE Database, Web of Science, and Scopus, utilising different “key words” to order identify studies investigating potential associations between exposure to OCPs and development of recurrent PCa following treatment (Figure 1).
MeSH controlled vocabulary was utilised, including combinations of the following key words: ‘‘organochlorine pesticides’’, ‘‘exposure’’, ‘‘DDT’’, ‘‘DDE’’, ‘‘hexachlorocyclobenzene’’, ‘‘lindane’’, ‘‘chlordecone’’, ‘‘kepone’’, ‘‘chlordan’’, ‘‘dicofol’’, ‘‘mirex’’, ‘‘dieldrin’’, ‘‘endrine’’, ‘‘aldrine’’, ‘‘PCB’’, ‘‘dioxine’’, ‘‘endosulfan’’, ‘‘heptachlor’’, ‘‘methoxychlor’’, ‘‘toxaphene’’, ‘‘prostate cancer’’, ‘‘biochemical recurrence’’, ‘‘biochemical failure’’, ‘‘prostatic carcinoma’’, ‘‘prostatic neoplasm’’, ‘‘prostatic adenocarcinoma’’, ‘‘case – control studies’’ and ‘‘cohort studies.’’
Extracted domains included sttudy design, demographics, findings
An overview of five available studies investigating a potential relationship between exposure to OCPs and development of recurrent PCa following radical treatment is provided in Table 1. Two studies did not observe any significant relationship between the exposure of American Veterans to Agent Orange (AO) and subsequent BCR following radical prostatectomy19,20. Li et al. reported that exposure to AO significantly increased the Dioxin-TEQ level in blood samples (p < 0.001), but high dioxin-TEQ levels were not associated with an increased risk of subsequent BCR (p=0.23). A study by Ovadia et al. found that men exposed to AO did not have an increased risk of BCR following radical prostatectomy in both a univariate analysis (HR 1.03; 95% CI 0.84 – 1.25; p=0.80) and a multivariate analysis (HR 1.21; 95% CI 0.99–1.49; p=0.07). However, a study by Shah et al. reported a significant positive association between exposure to AO and BCR following radical prostatectomy. In this study of 206 men, those with documented exposure to AO had a significantly increased risk of subsequent BCR following radical prostatectomy (RR 1.55; 95% CI 1.15 – 2.09; p=0.004 when adjusted for clinical characteristics, and RR: 1.47; 95% CI 1.08 – 2.00; p=0.02 when adjusted for clinical plus pathological characteristics)21. Another study by Brureau et al. revealed a significant positive association between exposure to Chlordecone and BCR following radical prostatectomy and no associations for DDE or PCB-135. In this study of 326 men, those with documented exposure to Chlordecone had a significantly increased risk of subsequent BCR following radical prostatectomy (adjusted HR = 2.51; 95% CI: 1.39 – 4.56; for the highest versus lowest quartile of exposure; p trend = 0.002). In addition, sensitivity analysis revealed that Chlordecone exposure was still significantly associated with a risk of BCR after excluding patients with positive surgical margins or prostatectomy ISUP Gleason grade 3 or higher, or advanced pathological stage22.
| Author | Population study | Exposure characteristics | Definition of BCR | Association with BCR |
|---|---|---|---|---|
| Li et al. Prostate cancer and prostatic diseases (2013)19 | 93 men (37 with pesticides exposure and 56 without) from American Veterans administration treated by radical prostatectomy for prostate cancer between April 2005 and September 2009 with a median follow-up of 5.3 years | 37 men were exposed to Agent O during the Vietnam War. Exposure evaluation by measurement Dioxin-TEQ level in blood. | Not defined | No significant association |
| Ovadia et al. Urologic Oncology: Seminars and Original Investigation (2015)20 | 1882 men (333 with pesticides exposure and 1549 without) from American Veterans administration treated by radical prostatectomy for prostate cancer between 1998 and 2011 with a median follow-up of 85 months | 333 men were exposed to agent O during the Vietnam War. Agent O exposure was determined by veteran self-reported verified by Veterans Affairs committee in order to determine Veterans exposure according to his location during the war. | 1 PSA level > 0.2 ng/ml, 2 levels of 0.2 ng/ml or secondary treatment for a detectable PSA after radical prostatectomy | No significant association |
| Shah et al. British Journal of Urology International (2009)21 | 1495 men (206 with pesticides exposure and 1289 without) from the Shared Equal Access Research Cancer Hospital database Veterans Affairs treated by radical prostatectomy for prostate cancer between 1988 and 2007 with a median follow-up of 49 months. | 206 men were exposed to agent O during the Vietnam War. Agent O exposure was determined by veteran self-reported | Biochemical progression was defined as one PSA level of > 0.2 ng/mL, two of 0.2 ng/mL, or secondary treatment for an elevated PSA level after radical prostatectomy. | Significant association |
| Brureau et al. International Joural of Cancer (2020)22 | 326 men with pesticides exposure from University Hospital of Guadeloupe (FWI) treated by radical prostatectomy for prostate cancer between 2004 and 2007 with a median follow-up of 73 months. | 326 men were exposed to Chlordecone, PCB 135 and DDE | Biochemical progression was defined as one PSA level of > 0.2 ng/mL, two of 0.2 ng/mL | Significant association for Chlordecone No significant association for DDE and PCB 135. |
| Author | Study population | Exposure characteristics | Advanced disease | Association with metastatic disease |
| Koutros et al. Environmental Health Perspectives (2015)22 | Case control study included 150 cases and 314 controls in a population-based cohort in Norway, who were diagnosed from enrolment through 31 December 1999 and were diagnosed at least 2 years after serum collection. | 150 men with metastatic prostate cancer from Janus Serum Bank cohort in Norway. 11 organochlorine pesticides and their metabolites and 34 PCB congeners were analysed | Metastasis and histologic grade were caracterized according to the American Cancer Society’s. All 184 incident metastatic prostate cancer cases didn’t have history of cancer (except non melanoma skin cancer) | Significant association for: Oxychlordane. PCB44 (inverse association) |
A report by Koutros et al. suggests that other pesticides, such as Oxychlordane and PCB44, may be implicated in modifying the risk of developing advanced PCa. For example, the development of metastatic PCa was twice as likely among men with a serum concentration of Oxychlordane in the highest quartile when compared against those in the lowest quartile (OR 2.03; 95% CI 1.03 – 4.03; p-trend=0.05). Findings for specific PCB-related chemicals showed a significant inverse association between natural log–transformed lipid-adjusted PCB44 and metastatic PCa (OR 0.74; 95% CI 0.56–0.97; p-trend=0.02)23. All characteristics of OCPs involved in BCR or metastatic PCa are summarised in Table 2.
| Name | Molecular structure | Comments |
|---|---|---|
| Agent Orange contaminated by TCDD (2,3,7,8-tétrachlorodibenzo- para-dioxine) |  | Lipophilic molecule hence its stability when it’s in a living organism. It’s resistant to the mechanisms of detoxification and remain stored in the adipose tissue of animals. It’s chemically very stable molecule and is therefore bio-accumulated. His half-life is 5–10 years in human body25. |
| Oxychlordane |  | Because of their lipophilic properties and their persistence in the environment, chlordane and related compounds bioaccumulate and biomagnify along the food chain26. |
| PCB44 2,2′,3,5′-Tetrachlorobiphenyl | 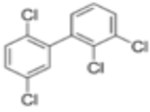 | PCBs appeared to early twentieth century chemists interesting for their dielectric properties. These are ubiquitous and persistent pollutants. Highly fat soluble, they are part of the bioaccumulative contaminants commonly found in fatty tissue in humans. Food is the primary source of PCB exposure. They have endocrine disruptor properties27. |
| Chlordecone | 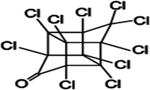 | Chlordecone interferes with estradiol signaling through binding to the nuclear estrogen receptors α (ERα) and β (ERβ), eliciting agonistic and antagonistic effects, respectively28. |
This systematic review confirms that there is a relative lack of high-quality evidence implicating a potential association between exposure to OCPs and BCR of PCa. However, the available evidence suggests that there may be a potential causal relationship between exposure to OCPs and development and progression of this malignancy. Agent Orange is the most widely-studied OCP in the context of any possible causal role in the recurrence of PCa following radical prostatectomy, or in the progression to advanced disease24. However, only two studies demonstrated a significant association between exposure to OCPs and subsequent BCR following radical prostatectomy. Two pesticides were involved: Chlordecone and Agent Orange21,22. Another study by Koutros et al. identified a significant association between exposure to Oxychlordane and PCB44 and progression to advanced PCa23. However, each of these studies are limited by their inclusion of a relatively small number of cases. Larger prospective clinical studies would be necessary to confirm these potential associations, however it is recognised that such studies would be very difficult to conduct, and are not presently feasible.
This review highlights the relative lack of evidence on the potential causal role of OCPs in PCa development and progression, despite the observation that a large number of pesticides exist and continue to be in use in many countries worldwide (Table 3). As such, this topic has potential impacts in aspects of global healthcare, and there is widespread public concern regarding pesticide exposure and negative impacts on health29. There is a well-documented causative relationship between exposure to pesticides and increased risk of development of many types of malignancy. It is therefore important to understand in greater detail the potential influence of OCP exposure upon aspects of PCa risk, and to identify the molecular pathways and mechanisms implicit in this increased risk (Figure 2).
| References | Study design | Pesticides | OR (95% CI) | Intensity of exposure |
|---|---|---|---|---|
| Alavanja et al. American Journal of Epidemiology (2003)27 | Cases-Controls 566 cases and 54766 controls | Chlorinated pesticidesa | 1.3 (1.0 – 1.6) 1.5 (1.2 – 2.0) | T2 T3L |
| Mills et al. Journal of occupational and environmental medicine (2003)15 | Cases-Controls 222 cases and 1110 controls | Lindane Heptachlor | 1.9 (1.1 – 3.2) 2.4 (1.2 – 4.6) 2.1 (1.2 – 3.5) 2.0 (1.1 – 3.6) | Level 3 Level 4 Level 3 Level 4 |
| Settimi et al. International journal of cancer (2003)28 | Cases-Controls 124 cases and 659 controls | DDT Dicofol | 2.2 (1.1 – 4.8) 2.8 (1.5 – 5.0) 2.8 (1.5 – 5.0) 2.4 (1.2 – 5.3) 3.0 (1.3 – 7.0) | Ever ≤ 15 years Ever ≤ 15 years > 15 years |
| Xu et al. Environmental Health Perspectives (2010)24 | Cases-Controls 1153 cases and 3999 controls | beta-Hexachlorocyclohexane | 3.4 (1.2 – 2.9) | T3U |
| Band et al. The Prostate (2011)16 | Cases-Controls 65 cases and 1920 controls | DDT Lindane | 1.7 (1.0 – 2.7) 2.0 (1.2 – 3.6) | High High |
| Cockburn et al. American Journal of Epidemiology (2011)29 | Cases-Controls 173 cases and 162 controls | Organochlrorine pesticidesb | 1.6 (1.0 – 2.6) 2.0 (1.2 – 3.5) | Ever High |
| Multigner et al. Journal of Clinical Oncology (2011)17 | Cases-Controls 623 cases and 671 controls | Chlordecone | 1.8 (1.2 – 2.6) | Qu4 |
| Emeville et al. Environmental Health Perspectives (2015)18 | Cases-Controls 576 cases and 655 controls | DDE | 1.5 (1.0 – 2.3) | Qu5 |
Some OCPs, such as PCBs and Chlordecone, have functional properties that disrupt various endocrine pathways, including the synthesis, secretion, transport, and binding of hormonal ligands to their cognate receptors, whilst in addition they may result in the elimination of natural human hormones30. Phthalte pesticides are endocrine disruptor molecules with demonstrable estrogenic effects in breast and PCa cells, and these may also be implicit in the etiology of hormone-independent PCa cancer31. Given that phthalates are estrogen-like substances, they can positively regulate the proliferation of human hormone dependent PCa cells by acting on the crosstalk between TGF-β and oestrogen receptor signaling pathways32. In addition, some studies suggest that estrogen and xenobiotic carcinogens may play an important role in PCa progression via oxidative estrogen metabolism. For example, the CYP1B1 enzyme is involved in the hydroxylation of estrogens, and this reaction is of key relevance to the regulation of estrogen metabolism33. The over-production of estrogen-like E2, or the bioconversion of E2 into genotoxic metabolites such as estradiol-3,4-quinone or 4-hydroxyestradiol by CYP1B1, may lead to the generation of reactive oxygen species which subsequently cause DNA damage and enhance PCa progression34. In support of this hypothesis, Gu et al. observed that men with the CY1B1 rs1056836 CC genotype had an increased risk of PCa recurrence following radical prostatectomy when compared against a combined CG and GG genotype35.
In conclusion, this review highlights the relative lack of studies regarding the potential influence of OCPs on the recurrence and progression of PCa following radical therapy. An increased understanding of the pathways and mechanisms through which pesticides may influence the natural history of PCa progression could influence the clinical management of men with this ubiquitous and common malignancy. It is important that the current relatively small body of evidence demonstrating a negative influence of OCPs on PCa risk should be added to in as timely a fashion as possible so that knowledge of this important health topic can increase, with a resultant positive health benefit for a significant number of individuals worldwide.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: PRISMA checklist for ‘Organochlorine pesticide exposure and risk of prostate cancer development and progression: a systematic review’, https://doi.org/10.6084/m9.figshare.14245694.v136.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Iwasaki M, Itoh H, Sawada N, Tsugane S: Exposure to environmental chemicals and cancer risk: epidemiological evidence from Japanese studies.Genes Environ. 2023; 45 (1): 10 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental Carcinogenesis and hormone-dependent cancers
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Prostate cancer - clinical research
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 01 Apr 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)