Keywords
myocarditis, myositis, immunotherapy, checkpoint inhibitor, adverse events
myocarditis, myositis, immunotherapy, checkpoint inhibitor, adverse events
Myocarditis is a rare but feared adverse event of treatment with immune checkpoint inhibitors (ICI). The incidence is about 1%, while the mortality rate is up to 50%. The incidence of lung cancer in Norway is 117 per 100 000 with 3200 patients a year. 30% of patients are treated with surgery or stereotactic radiation. The remaining patients are eligible to treatment with ICI in first or later lines. ICI has been used for lung cancer in Norway since 2016. Still, most physicians in Norway treating lung cancer have not experienced ICI-induced myocarditis. An informal query to six university hospitals and eight local hospitals in the South-East Health Region in Norway revealed no routine examination (blood or imaging) at baseline to help detect development of ICI-induced myocarditis later. An electrocardiogram (ECG) is usually part of the diagnostic work-up before cancer treatment, but only two hospitals specify taking ECG before start of immunotherapy in later lines. Our impression is that the situation is similar in the other Nordic countries (after personal communication through the Nordic Cardio-Oncology Board). The literature indicates the importance of early diagnosis, as a retrospective study found favourable outcome with onset of corticosteroid treatment within 24 hrs of admission to hospital1. We present this case to increase awareness, to advocate early diagnosis and treatment of ICI-related myocarditis.
A woman in her late sixties, working freelance without any exposure and never smoking, with paroxysmal atrial fibrillation and a subvalvular aortic stenosis had a right upper lung lobectomy for an epidermal growth factor receptor (EGFR) positive adenocarcinoma. Post-surgery, she was treated with adjuvant chemotherapy and radiotherapy. A relapse two years later was treated with erlotinib and paused after a year due to gradual progression. After 18 months, upon progression, carboplatin/pemetrexed/pembrolizumab was started. When seen for the second infusion, she had slightly increased dyspnoea and was feeling tender on palpation of the chest wall medial of her right breast and the food felt as if it stopped in her oesophagus if she ate too quickly. There was no cough or fever. Clinical examination, c-reactive protein (CRP), haematology and electrolytes were normal. Troponins and creatine kinase (CK) were not measured. Computerized tomography (CT) showed no signs of pneumonitis and carboplatin/pemetrexed/pembrolizumab treatment was continued.
Two weeks later she was admitted to the oncology department with increased dyspnoea, general body ache, muscular weakness, dysphagia, episodes of palpitations and diplopia. Clinical examination revealed ptosis. Her blood work-up showed troponin T 651 (<14) ng/L, NT-proBNP 6761 (<760) ng/L, CRP 6.4 (<5) mg/L, aspartate aminotransferase (AST) 453 (<35) U/L (grade 3 toxicity), alanine aminotransferase (ALT) 186 (<45) U/L (grade 2), lactate dehydrogenase (LDH) 1111 (<255) U/L and creatine kinase (CK) 5845 (<210) U/L. Her ECG showed sinus rhythm and a new left bundle branch block (Figure 1A). CT excluded pulmonary embolism. Three days later she developed a third-degree atrioventricular block (Figure 1B) and her clinical symptoms progressed. Echocardiography showed no regional wall motion abnormalities. Cardiac magnetic resonance imaging (CMR) was attempted, but aborted as the patient was unstable because of her third-degree atrioventricular block. A two-chamber pacemaker was implanted and a coronary angiography was done and showed no signs of acute coronary syndrome. Later, repeated echocardiography was unchanged from the initial echocardiography, the left ventricular ejection fraction (LVEF) continued to be normal of 58%. With the presenting symptoms, troponin elevation, third degree atrioventricular block and no significant stenosis on the coronary angiogram, the patient fulfilled the criteria of myocarditis2 and it was concluded the patient had ICI-induced myocarditis with myositis and myasthenia gravis-like syndrome.
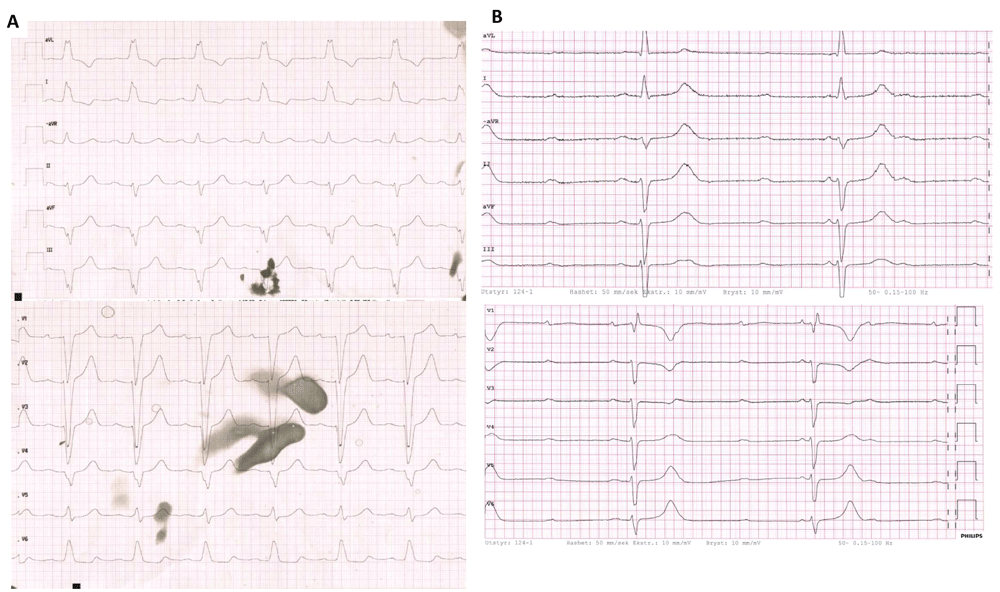
Electrocardiogram at admission after the second infusion of combination therapy (A) and three days after admission (B).
Intravenous methylprednisolone 2 mg/kg/day was initiated on day 3 and increased to 1000 mg/day for four days as her troponins increased (Figure 2). As there was a lack of clinical and biochemical response, abatacept 1000 mg once every second week and oral mycophenolate mofetil 750 mg b.i.d was initiated. This improved the biochemical parameters, but the clinical symptoms were unchanged. On day 14 plasmapheresis with exchange of 2l plasma was performed, but could not be repeated as the patient did not tolerate the exchange. However, the biochemical values improved further and her ptosis, dyspnoea and general muscle weakness improved. Unfortunately, her dysphagia and dyspnoea kept deteriorating. On day 25, abatacept and mycophenolate mofetil was stopped and the patient died five days later.
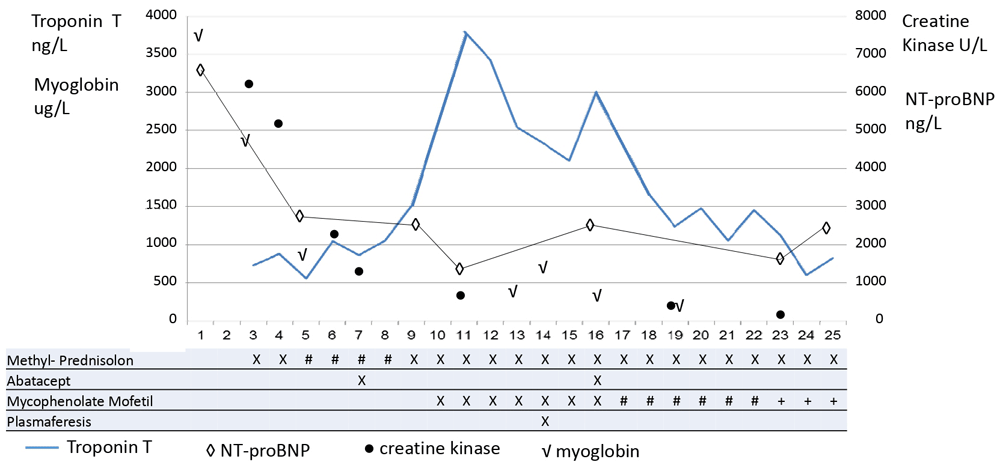
Serum levels of troponin T increase during the first 10 days. Normalization of creatine kinase and myoglobin may reflect improvement of myositis, while the myocarditis did not resolve. Daily treatment regimens are indicated as follows: methylprednisolone X= 2 mg/kg, #= 1000 mg × 1; abatacept X=20 mg/kg; mycophenolate mofetil X= 750 mg × 2, #=500 mg x 2, +=500 mg × 1.
Myocarditis is a rare incidence (0.06-1.14%)3,4 off-target effect of ICI with mortality rates up to 50%5. The symptoms are often non-specific as fatigue, dyspnoea or chest pain. In severe cases cardiac arrhythmias as atrioventricular block or ventricular arrhythmias can occur. The myocarditis can be combined with myositis and/or myasthenia gravis like syndrome6. With increasing use of immunotherapy alone and in combination with other therapies, an increase in immune mediated myocarditis can be expected. Combination immunotherapy has a higher incidence than distribution of a PD-1/PD-L1 inhibitor alone7. There are few studies reporting the incidence of myocarditis after combination of ICI and chemotherapy. Keynote 189 reported one case of myocarditis in the treatment arm with pembrolizumab, carboplatin and pemetrexed (of 405 patients)8.
As the incidence of myocarditis is rare, the symptoms non-specific and because in the acute setting, the patients are often seen by medical staff with less experience of immunotherapy off-target effects, the diagnosis may be missed unless there are routines for baseline examinations and pre-defined early diagnostic work-ups. As a minimum, before initiation of immunotherapy, ECG should be taken and troponins and NT-proBNP measured. There should be a low threshold for repeating these measures during treatment, particularly if the patient is admitted to the hospital. After onset of symptoms suspicious of myocarditis, coronary angiography can rule out acute coronary syndrome, while CMR can often detect myocardial oedema along with late gadolinium contrast enhancement (LGE) as a sign of inflamed myocardium. However, in a recent study of 103 patients with ICI-associated myocarditis LGE was only present in 48%, other signs of oedema as elevated T2-weighted short tau inversion recovery (STIR) were present in 28%. The presence of LGE or T2-weighted STIR were not associated with major adverse cardiovascular events (composite of cardiovascular death, cardiogenic shock, cardiac arrest and complete heart block)9. In our patient CMR was not performed as she was unstable because of a third-degree atrioventricular block and implantation of a pacemaker was prioritized. Diagnosis was based on the diagnostic criteria for clinically suspected myocarditis as recommended by the European Society of Cardiology (ESC)2. Early immune suppression is of the essence, and hence, clinical awareness and diagnostic routines are important. In a recent retrospective study, high dose methylprednisolone (1000 mg/d) and early initiation (<24h) were associated with improved cardiac outcomes1. Combination of several anti-inflammatory therapies and plasmapheresis may reverse the serious adverse event6,10.
Education of medical staff in various hospital departments and in the community is necessary and may increase awareness of ICI-associated myocarditis. Clear routines for standard investigations prior to and during treatment with immunotherapy may reduce time to diagnosis, increase awareness and hopefully reduce mortality of myocarditis.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
References
1. Suzuki S, Ishikawa N, Konoeda F, Seki N, et al.: Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan.Neurology. 2017; 89 (11): 1127-1134 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Melanoma, Immunotherapy, myelodysplastic syndrome
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Jan 21 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)