Keywords
Photodynamic therapy, porphyrin, Leishmania panamensis, Photophysical study, in vitro, porphyrinato
This article is included in the Pathogens gateway.
Photodynamic therapy, porphyrin, Leishmania panamensis, Photophysical study, in vitro, porphyrinato
We have made improvements to this version of the article thanks to the suggestions of reviewer 2, the changes realized are described below. We thank reviewer 2 for the suggested corrections, all were very accurate and solicited valuable information that improved this version of the article.
1. Methodology:
(a) added punctual information on the parasite inoculum used: inoculum of 5 x 10 6 cells/mL (promastigote).
b) information was provided on the type of microplates used: 96-well microplates.
c) relevant information was provided on incubation and irradiation times of the parasite cultures versus treatments: the assays were irradiated for 24, 24, and 72 hours.
2. Results:
a). We have corrected the range of photoactivation of the compounds; by mistake we had included 400-7000 nm, being correct 400-700 nm.
b). We have changed reference 53, for a more appropriate one from Valery Tuchin's.
c) In figure 5b, we provide details on the label about points I, II, and III and the solvent used.
d). the exact p-values obtained from the statistical comparisons are included.
e) We have included an item on the results of leishmanicidal activity of compounds 1 and 2, in addition, the statistical comparisons obtained where it is shown that the activities are similar.
3. Bibliographies: We changed reference 53, for a more appropriate one from Valery Tuchin's.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Porphyrins and metalloporphyrins are versatile macrocyclic organic compounds, from the structural viewpoint; the porphyrin main skeleton consists of four pyrrole rings bound through their alpha carbons (α-C) with four aldehydes.1,2 These structural characteristics confer on the porphyrins a variety of properties such as high conjugation, symmetry, and planarity. Additionally, they acquire the ability to complex with a large number of metals in their interior through the coordination of the four pyrrolic nitrogen atoms submerged in the molecule.3,4 The conjugated and aromatic structure of porphyrins allows interactions between π electrons and different metals, facilitating the binding to their coordination centers.5 Porphyrins have absorption and intense electronic emission at wavelengths greater than 400 nm, small energy for HOMO-LUMO transitions and the ability to adjust their optical redox properties.6-10 All these properties make porphyrins relevant macromolecules in several chemistry fields (e.g. material science, optics, catalysis, transformation and storage of energy, medicine, pharmacology).11-17 In recent years, porphyrins have emerged as important promising photosensitizers in photodynamic therapy (PDT); porphyrin and its derivatives demonstrate great efficacy as both antibacterial and antiviral agents against different species due to its exceptional photodynamic properties.18,19 Porphyrins have potential applications in biological sciences, therefore, it is pertinent to develop simple synthetic pathways that lead to compounds with unique physical and chemical characteristics.
Nowadays different strategies to improve porphyrin photodynamic properties have been applied: (a) structural modification of the base ring with the addition of a variety of substituents at messo position, and (b) the inclusion of metals into the porphyrin core.20-22 The photophysical properties of porphyrins and their metallic-derivatives are affected for both the peripherally and/or axial substituents and the central metal into porphyrin core.23 The porphyrins and their metallic-derivatives act efficiently as sensitizing agents and they have presented phototoxic activity against different pathogens, such as bacteria, fungi, viruses and parasites.24-27 Different porphyrinic photosensitizers have reported anti-leishmanicidal activity against Leishmania tarentolae in promastigote stage (ethyl and diethyl carbaporphyrin), against amastigotes of L. panamensis, and promastigotes of L. major and L. braziliensis (β-substituted porphyrinic systems).28-32 Improved pharmacological responses have been found when incorporating metals in the macrocycle, as reported by Gomes et al. when evaluating the activity against L. amazonensis against derivatives metalated with Bi (III) and Sb (IV) (IC50 of 93.8 μM and 52.4 μM respective).33 Another Zn (II) metalated porphyrin derivative evaluated against promastigotes of L. braziliensis reduced parasite viability with greater efficiency than the metal-free derivative.34 This type of modification alters the steric and electronic nature of porphyrins giving rise to new molecules that have specific and unique properties, and it also presents a promising alternative for modifying photophysical properties of the compounds: (a) quantum singlet oxygen performance, (b) the range of the therapeutic window, (c) photostability and (d) lipophilicity could be improved.35
Although, 5,10,15,20-tetrakis(4-bromophenyl)-porphyrin (compound 1) and dicloro-5,10,15,20-tetrakis(4-bromophenyl) porphyrinato Sn (IV) (compound 2) are commercially available, the reports about of its application as sensitizers in PDT are few. Therefore, in this study, we analyzed the photophysical behavior of (1) and (2) as regards their potential use in PDT against Leishmania panamensis.
All reagents and solvents were purchased from Sigma Aldrich. We prepare the porphyrin 5,10,15,20-tetrakis(4-bromophenyl) porphyrin (1) based on Adler’s method,36 introducing a small modification that consisted of leaving the reaction for 8 hours at room temperature and stirring in an open container, using the oxidative power of oxygen to convert more of the chlorin by-product into porphyrin. In summary, equimolar amounts of pyrrole and 4-bromobenzaldehyde were mixed in propionic acid for 8 hours at ambient temperature under an atmosphere of air. Dicloro-5,10,15,20-tetrakis(4-bromophenyl) porphyrinato Sn (IV) (2) was synthesized by porphyrin precipitation in metal chloride solicitation (Figure 1). The formation of the final products was followed by thin layer chromatography on aluminum foil UV254 TLC, the mobile phase was petroleum ether-ethyl acetate (2:1). The compounds were characterized using 13C NMR spectra (Bruker AC-400 spectrometer); the 13C NMR chemical shifts are reported as ppm (δ), relative to CDCl3 (signal located at 7.29 ppm). Infrared spectrum was measured on the equipment ECO-ART alpha Bruker FTIR spectrometer. To perform UV-Vis spectrum (using a UV-2401PC UV-Vis spectrophotometer), we dissolved 2.0×10−5 g of each compound in ethyl acetate, and finally, we obtained the mass spectrum by dissolving the compound in methanol (using ESI-LC-MS/MS ion Trap amaZon, Bruker spectrometer). Furthermore, we measured fluorescence quantum yield (using a PTI um 40 fluorimeter) and singlet oxygen quantum yield,37 and electrochemical characterization was performed in four different dissolvents (Dimethylformamide-DMF, dichloromethane -DCM, dimethylsulfoxide-DMSO, tetrahydrofuran-THF) containing 1.0×10−1 M tetrabutylammonium perchlorate ((C4H9)4N(ClO4), Aldrich 98% purity) as a supporting electrolyte for all electrochemical measurements.
Compound (1) was prepared by mixing 10 mmol pyrrole and 10 mmol 4-bromobenzaldehyde in 80 ml propionic acid for 8 hours at ambient temperature in an open container. The product was extracted from the reaction medium by adding 60 mL cold methanol and filtering by gravity, the filtrate was dried at room temperature, obtaining 1.4136 g of a purple solid that was finally purified using column chromatography (mobile phase; ether-ethyl acetate 20:2). Yield: 47.28%; melting point > 300°C; UV-Vis (ethyl acetate) 414, 512, 546, 591, 645; FT-IR (cm −1): N-H (3350), C=C (1470.28), C=N (1088.22), C-N (964.28); 13C NMR (CDCl3, 400 MHz): 118.86 (Ar), δ = 122.92 (Ar), δ = 130.22 (β-py), δ = 131.95 (Ar), δ = 135.95 (Ar ipso), δ = 140.91 (Ar-Br); (M+H) m/z = 930.9. These results concur with previous reports for this compound.37,38
Compound (2) was prepared by mixing 0.5478 mmol of (1) with 2.2164 mmol SnCl2.2H2O in DMF (80 mL) for 4 hours at ambient temperature, and by stirring. After that, we added cold water and TBrPP-Sn (IV) precipitated; this solid was washed and dried at ambient temperature. The compound was purified by column chromatography (mobile phase; petroleum ether-ethyl acetate 5:1). Yield: 64.4%; melting point > 300°C; UV-Vis: 426, 560, 600. These results concur with previous reports for this compound.37,39
Fluorescence quantum yield (ϕf) was determined by the comparative method in a PTI um 40 fluorimeter. Using as a standard fluorescein dissolved in water, porphyrin (1) and metalloporphyrin (2) was dissolved in ethyl acetate. All fluorescences were determined taking as excitation wavelength the maximum of the Soret band, using a 2 nm slit and a 420-750 nm scan. The fluorescence quantum yield was calculated with the following eq. 1.37,40,41
Fx and Fest are the areas under the fluorescence emission curve of compounds (1), (2) and the standard. A x and A are sample absorbances and standard at the excitation wavelength. ɳ x and ɳ are the respective refractive indices of the solvents (ethyl acetate; ɳ = 1.3724, water; ɳ = 1.33336).
Singlet oxygen quantum yield (Φ∆) of (1) and (2) was performed by the graphical method, using 1,3-diphenylisobenzofuran (DPDF) as singlet oxygen scavenger, and singlet oxygen generator standard 5,1,15,20-(tetraphenyl) porphyrin (H2TPP). The tests were carried out by preparing a 1×10-9 M solution of each compound in DMF in triplicate and calculated with eq. 2.37,40,41
Where: Φ∆standard is the singlet oxygen quantum yield of the H2TPP standard in DMF (0.64). W and Wstandard are the slopes of the degradation curves of the DPDF.
Cyclic voltammetry (CV) data were recorded using a single-compartment electrochemical cell with a maximum electrolyte volume of 10 mL. A CH Instruments (Model 600E) Electrochemical Analyzer was used for the electrochemical measurements. The working electrode was a glassy carbon 3 mm in diameter on Teflon R (CH Instruments). The reference electrode used was an Ag+/Ag electrode on Teflon R; we used a solution 1.0×10−3 M AgNO3 in electrolyte support; the auxiliary electrode was a platinum of 99.99% from CH Instruments. The electrochemical characterization was carried out using cyclic voltammetry and linear voltammetry. The peak current intensity, in cyclic voltammetry, is given by the Randles-Sevcik Eq.3:42
where is the number of electrons in the redox reaction, is the area of the working electrode, is the diffusion coefficient for the electroactive species, is the scan rate, and is the concentration of the electroactive species in the electrode. The anodic and cathodic peak currents are equal, and the ratio is 1.0. The half-wave potential, , is midway between the anodic and cathodic peak potentials, Eq. 4.
Leishmania panamensis (UA140) was used in in vitro tests for the evaluation of the leishmanicidal potential of the compounds (1) and (2). Leishmanicidal activity was determined as the ability of the compounds to decrease the viability of the parasite, for this the MTT method is widely used in the literature (MTT Assay Protocols, Thermo Fisher Scientific). Conditions were previously standardized by our working group.37,43-45
Leishmania panamensis (UA140) were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 1% glutamine and 1% antibiotics (200 U penicillin/200 μg Amikacin) under incubation conditions 5% CO2.37,45 The metacyclic promastigotes in the infectious stage were isolated from stationary cultures. The parasite viability was estimated by MTT assay.37,43-45 Anti-leishmanicidal activity was evaluated at different concentrations (1, 10, 50, 100 and 200 μM) of compound and positive control (Glucantime), against a parasitic inoculum of 5 × 106 cells/mL (promastigote). Test compounds and positive control were dissolved in dimethyl sulfoxide (DMSO), working concentrations were obtained by adding 10 µL of compound in a final volume of 200 µL to each well of the 96-well microplate, the treatments were maintained under visible light irradiation, with incubation times of 24, 48 and 72 hours. The irradiation source was Omnilux lamps (EL10000AG), with a range λ emission lamp = 420 nm–450 nm, and an incident photon flow per unit volume Io was 5.7 × 10−7 Einstein*L−1s−1. Each trial was performed in triplicate. Plates were analyzed using SkanIt software. We applied an ANOVA test to determine the differences or similarities between treatments and positive control. In addition, a post hoc analysis was performed using Tukey statistics. Finally, differences were considered to be significant when p < 0.05.
The UV-Vis spectrum of (1) (Figure 2), shows a band of maximum absorption located at 414 nm (Soret band), generated by a1u(π)-eg*(π) transitions and four lower absorption Q band located at 515nm, 547nm, 588nm and 645 nm, which corresponds to a2u(π)-eg*(π) transitions.46,47 The UV-Vis spectrum of compound (2) shows one Soret band and only two Q bands. When the Sn (IV) ion coordinates nitrogen atoms inside the porphyrin ring, the porphyrin symmetry increases. Furthermore, the reduction in the number of Q bands indicates that the metal effectively entered the macrocycle.48 The intensity of the Q bands is correlated with the relative stability of the metalloporphyrin: when the signals are of low intensity, the metallocomposites are highly stable and their atoms are located in the square plane.49,50 Ohsaki et al. reported a similar change in the UV-Vis spectrum after tin (IV)-insertion into the porphyrin core synthesized in water at ambient temperature.51 Moreover, the Soret band for (2) had red shift from 414 nm to 425nm (near to 11 nm). The direct coordination between Sn (IV) ion and porphyrin core could extend conjugation from porphyrin to metal ion; in this case, the electronic excitation will require lower energy absorption due to increasing conjugation–this process requires longer wavelength than pure porphyrin.10,52 Figure 2 shows that (1) and (2) have photo-activity inside window 400 to 700 nm. Although the compounds do not have a considered absorption in the red rank of the visible light spectrum from 600 nm to 800 nm (this radiation can reach a penetration depth of 8.0 mm inside tissue), they absorb radiation inside range 500-600 nm. This radiation penetrates approximately 4.0 mm, and such penetration capacity is suitable for potential application in cutaneous treatments.53
Deamination of Փf
Information related to the efficiency of fluorescence emission is important to explain the inactivation pathway related to PDT.54 Figure 3 shows fluorescence emission for (1) and (2); both show photoluminescence at a visible range in the electromagnetic spectrum. As shown in Figure 3, fluorescence emission wavelength was located at 651 nm for both compounds, the energy transition did not change after the Sn (IV) ion insertion; however, fluorescence emission intensity was more high compound (1). This effect is due to the Sn (IV) ions insertion inside the porphyrin core decreases significantly fluorescence effect. As shown in Table 1, the Φf of (1) was three times greater than (2); the Sn (IV) ions inside the porphyrin core could increase the non-radiative decay of the excited singlet state of porphyrin,37 Sn (IV) ions within the porphyrin nucleus could increase disintegration by inter-system crossover (ISC), and this pathway is governed by orbital spin coupling at the central atoms; in this case, the insertion of Sn (IV) reduces the fluorescence emission.9,55,56 A similar effect was reported for cupper insertion inside meso-porphyrinic complexes.56,57
| Compound | λabs | λem | Φf | ΦΔ | ||||
|---|---|---|---|---|---|---|---|---|
| Soret Band | Q Bands | |||||||
| (1) | 414 | 512 | 546 | 591 | 645 | 651 | 0.15 ± 0.01 | 0.55 ± 0.03 |
| (2) | 426 | 560 | 594 | --- | --- | 651 | 0.05 ± 0.01 | 0.59 ± 0.04 |
Deamination of ՓΔ
The generation of singlet oxygen produced by (1) and (2) was quantified by chemical entrapment using DPBF. To estimate the ΦΔ, the degradation of DPBF was measured at 415 nm over time (Figure 4), the lower the absorbance, the greater the degradation of DPBF mediated by singlet oxygen. The complex Sn (IV)-porphyrin (2) presented higher ΦΔ compared to (1) (Table 1), the difference in ΦΔ between the compounds is 7%, and this difference is directly attributed to the insertion of the metal ion Sn (IV) inside the macrocycle. Sn (IV) would be generating greater stability of the triplet state of the molecule and improving the interaction with molecular oxygen, which is reflected as a greater translocation of the molecular oxygen spin and its subsequent conversion into singlet oxygen.4,59-62 These sensitizers show promise in PDT for its ΦΔ values, and could in the future be candidates in clinical trials like its counterpart Lutetium Texaphyrin, which has a ΦΔ as low as 0.11.63
The biological systems present microheterogeneity, caused by the coexistence of microphases such as aqueous polar and highly hydrophobic lipid.64,65 Therefore it is relevant to study the physicochemical properties of sensitizers at different media. We have studied the electrochemical behavior of (2) using cyclic voltammograms (CV) in four different solvents. Figure 5 shows CVs and Table 2 lists electrochemical parameters. Porphyrin had irreversible one-electron oxidation at Epa, which varies between 0.55 V for DMF and 1.0 V for THF, in addition to one quasi-reversible reduction peak between −1.01 V for THF and −1.41 for DMF (Figure 5a). The latter is clearer when DMF and DMSO are solvents. CVs (in detail) in DMSO, presenting three redox processes: (a) oxidation processes (I and II) related to the formation of monocationic and dicationic porphyrin spaces, (b) (a) oxidation processes (I and II) related to the formation of monocationic and dicationic porphyrin spaces, (b) reduction process (III) that results in the formation of the anionic porphyrin species66 (Figure 5b).
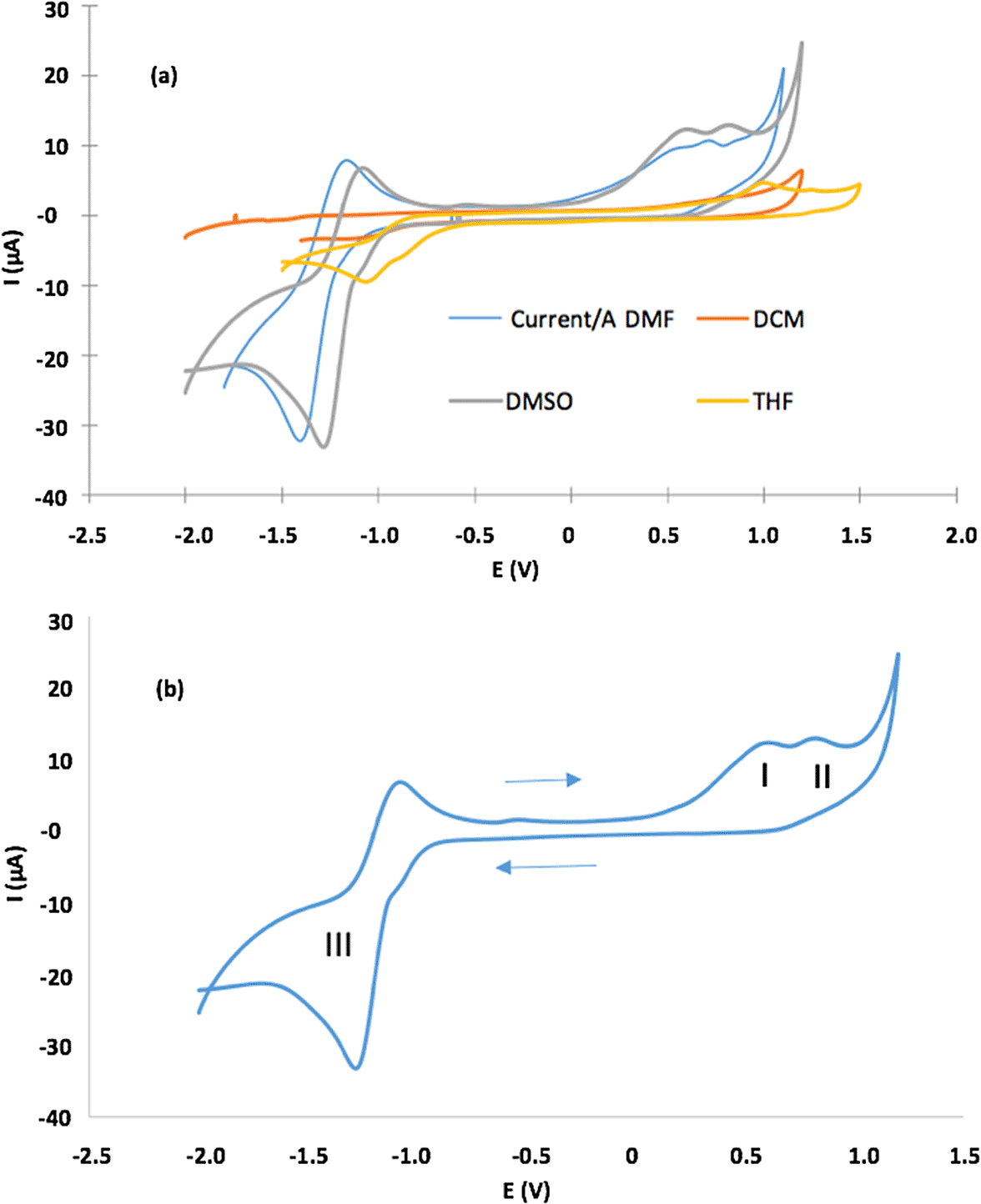
In the figure 5b, I and II correspond to oxidation processes that form mono- and di-cationic porphyrin species, and III corresponds to reduction processes that form anionic porphyrin species in DMSO as solvent.
| Solvent | Oxidation | Reduction First | ΔE1/2 | |
|---|---|---|---|---|
| Second | First | |||
| DMF | 0.71 | 0.54 | −1.41 | 0.98 |
| DCM | – | 0.83 | −1.11 | 0.97 |
| DMSO | 0.81 | 0.59 | −1.27 | 0.93 |
| THF | 1.25 | 1.00 | −1.01 | 1.01 |
The conformational and structural changes observed in reversible electron transfer reactions can be examined through the potential differences between the first and second oxidation states.46,67 Table 2 shows the oxidation and reduction potentials. The couple III presented an almost reversible behavior accompanied by a separation of anodic-cathodic peaks ΔEp> 60 mV, in addition, it presented an anodic-cathodic peak potential ratio of approximately unity (1.0). This result is characteristic of a nearly reversible monoelectronic process. The electron-attractant character of the bromine substituents could be significantly influencing the electrochemical properties of the derivatives (1) and (2).68,69 Likewise, Table 2, shows a slight effect on ΔE1/2 by change of dissolution solvent. THF had the highest ΔE1/2 due to lower dielectric constant value (ε), and this solvent had smaller dielectric constant value (εDMSO = 46.70, εDMF = 36.70, εDCM = 8,93 and εTHF = 7.58) between all aprotic dipolar solvents under study herein. Our data on the potentials for oxidation and reduction of TBrPP-Sn (IV) (Table 2) are consistent with previous reports published in the literature on metalloporphyrin-like compounds.70,71
We studied the scanning speed effect on the current response of CVs for each solvent to determine if the redox process was controlled by diffusion or by adsorption. Figure 6a shows Cvs for (2) at different scanning speeds in DMF. Figure 6a shows an anode peak for all scan rates in the range 0.8-1.3 V for all solvents used. The relationship found between the scanning speed and the peak current was directly proportional with linear increase, and the peak potential anodically shifted; additionally, when the scanning speed was increased, the peak became broader. Figure 6b shows that the peak current correlated with the square root of the scan rate for each solvent studied. Table 3 shows R2 and linear equation fitting for each test. The fitting results indicated that the process is controlled by diffusion, then hydrodynamics of media (e.g. polarity, density, viscosity) determines the redox process rate.66-68 Furthermore, the linear fit of the line plot of Ip versus v1/2 indirectly indicates a relationship between the diffusion coefficient and DMSO; and DMF had the highest slope value, suggesting that the diffusion coefficient for these solvents was greater than the diffusion coefficient for THF and DCM. This result is associated with the value of the dielectric constant, as discussed.67,68

| Solvent | Linear equation | R2 | slope) (μAV-1/2s1/2) |
|---|---|---|---|
| DMF | y=4.22×10−5x−8.1×10−7 | 0.956 | 4.22 |
| DCM | y=3.47×10−5x−8.1×10−6 | 0.997 | 34.7 |
| DMSO | y=5.29×10−5x−3.6×10−6 | 0.995 | 52.9 |
| THF | y=3.21×10−5x−6.9×10−6 | 0.992 | 32.1 |
Figure 7 shows dissolvent effect on electrochemical band gap value (2) for solvents studied in this work. The data obtained for the redox potentials (pH = 7.0 and room temperature) of the water separation reaction and the carbon dioxide reduction reactions to produce methane and methanol: Potential per redox couple; E (H2O/O2) = −5.26 eV; E (H +/H2) = −4.03 eV; E (CO2 / CH4) = −3.79 eV; E (CO2/CH3OH) = −3.65 eV.72 It is evident that the value of the electrochemical band gap depends on the polarity of the solvent, (2) had the smallest defective gap in THF. A requirement for the photosensitizer in PDT is the band gap; electrochemical characterization indicates that it is appropriate and suitable. Finally, the potentials described in Table 2 corresponding to the redox process of (2) are in agreement with other reports for processes based on rings in porphyrin complexes.72,73
A wide variety of molecules have been evaluated as possible therapy against Leishmania spp in recent years: (i) aluminum and zinc phthalocyanines (ii) methylene blue, 5-aminolevulinic acid and porphyrin. The field of research on new substances with challenging properties in medicine and pharmacology is an important topic, which has become more relevant due to the appearance of resistant and emerging microorganisms.74-76 The compounds (1) and (2) have been used to evaluate their effects on the viability of L. panamensis using the MTT method. The results are presented as cell viability of L. panamensis after exposure to different concentrations of (1) and (2) during incubation periods of 24, 48 and 72 hours, in darkness and under irradiation. The same procedure was done for the positive control (Glucantime).
The results show that (1) and (2) presented inhibitory activities on parasite viability (Figure 8). The decrease in the viability of L. panamensis was observed to a greater degree on the irradiated tests, this is due to the increased interaction capacity of the test compounds with oxygen, which induced the production of singlet oxygen.77,78
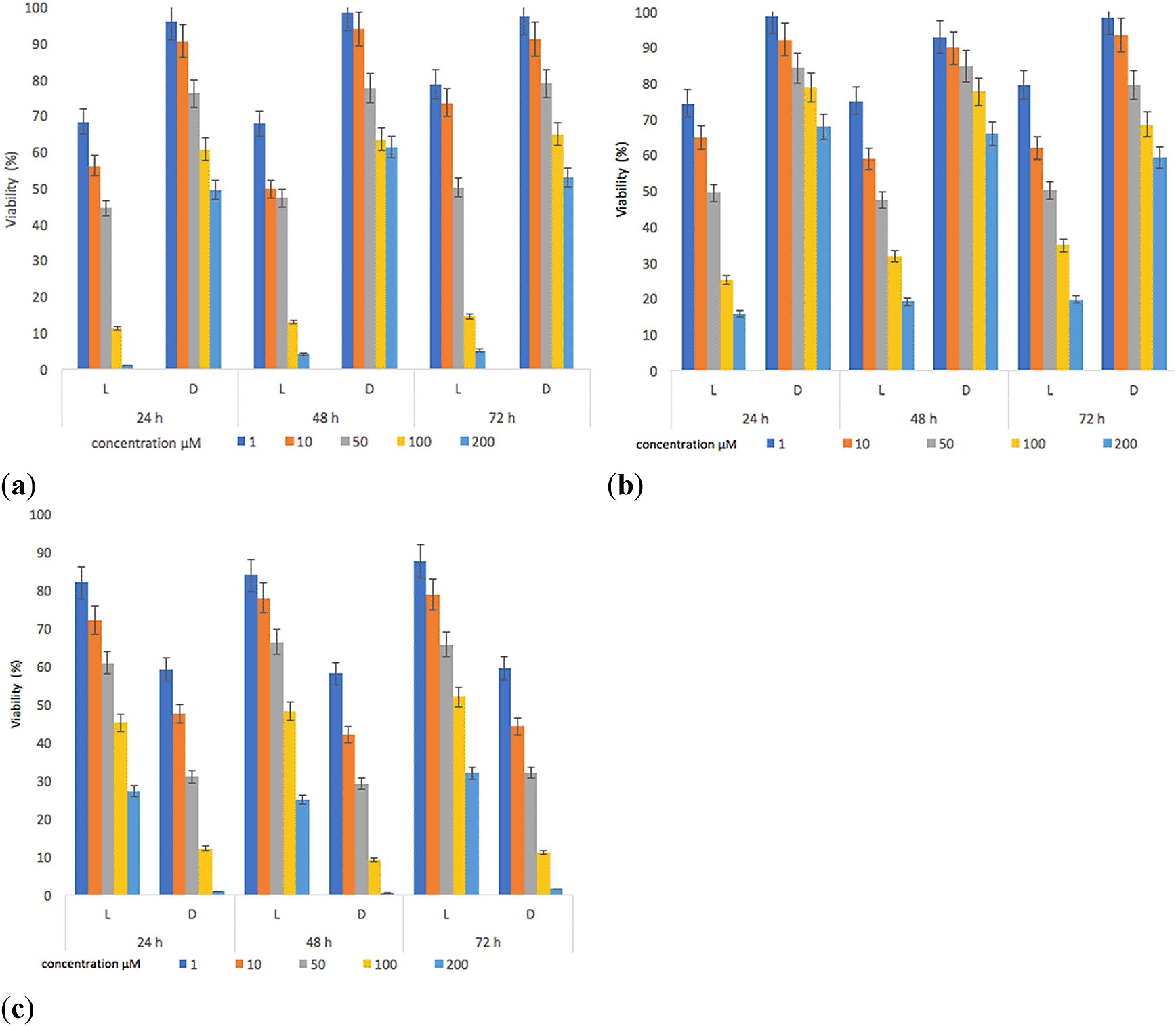
The contrary effect was evidenced for the reference standard, which reached better inhibitory activities on parasite viability in darkness. The highest effect leishmanicide was observed when the parasite was exposed to light for 24 hours and with concentrations of the compounds higher than 100 μM. Furthermore, the irradiation time (48–72) had no significant effect on viability (%) results compared to samples irradiated for 24 hours. The IC50 (Table 4) was determined using data found in the 24-hour test. Under irradiation, the IC50 of both compounds was lower compared to the positive control (Glucantime), with values of 16.5μM (p = 0.02) and 19.5 μM (p = 0.03) respectively. Additionally, we found slight differences between the activity of compound (1) and (2); compound (1) presented an IC50 = 16.5 μM vs IC50 = 19.2 μM of compound (2), however, these differences were statistically non-significant (p = 0.74). Therefore, these two compounds have similar behaviors in terms of parasite inhibition, which correlates with their ՓΔ values (ՓΔ compound 1 = 0.55 and ՓΔ compound 2 = 0.59). The activation of sensitizers by light action ensures lower IC50 values. Besides, in the absence of light, the response was lower, thus being in line with findings of other reports.78 Our results also show that (1) and (2) cause some damage to the parasite, decreasing its survival rate by 1.5-fold compared to the standard in the presence of irradiation, which could induce a reduction in the healing time of a lesion.
In the present study, we synthesized and characterized (1) and (2). Compound structures were confirmed by spectroscopic techniques (UV-Vis, FT-IR, 13C- NMR and ESI-mass). Sn (IV) ion insertion inside in the porphyrin core reduced significantly Փf 0.15 to 0.05. Furthermore, ΦΔ increased from 0.55 to 0.59 after metal insertion inside the porphyrin core. Electrochemical results showed that electrochemical properties were affected by the solvent dielectric constant, where THF had the highest ΔE1/2 due to a lower dielectric constant value. Moreover, the electrochemical assay showed a quasi-reversible reduction peak between −1.01 V in THF and −1.41 in DMF. The inhibitory results shown that (1) and (2) presented inhibitory activities on parasite viability. The highest inhibitory activity on the parasite was observed when the treatments were irradiated for 24 hours and with concentrations of the compounds of 200 μM, strengthening the hypothesis that parasite mortality is mediated by reactive oxygen species (especially singlet oxygen). These compounds were synthesized with low-cost methods and with acceptable synthesis yield, a fundamental aspect in the search for sensitizer candidates. The results of biological activity suggest these compounds could be applied in future applications of in vivo models as potential sensitizers of photodynamic therapy.
Mendeley Data: Complementary material, http://dx.doi.org/10.17632/h2vmrdz4sg.1.79
This project contains the following underlying data:
- 1. UV-Vis compound (1) and (2).xlsx [UV-Vis spectrum of compound 1 and 2]
- 2. Gráficos, RENDIMIENTO CUÁNTICO DE FLUORESCENCIA.xlsx [Graphics, Quantum fluorescence yield of compound 1 and 2]
- 2.1 Calculo de Qf.xlsx [Calculation of Quantum fluorescence yield (Qf) of compound 1 and 2]
- 3. 1. Compound 1 (DMSO). Rendimiento Cuántico Oxígeno Singulete – copia.xlsx [Calculation of Quantum Yield Oxygen Single of Compound 1 in DMSO]
- 3.2. Compound 2 (DMSO). Rendimiento Cuántico Oxígeno Singulete.xlsx [Calculation of Quantum Yield Oxygen Single of Compound 2 in DMSO]
- 4. ESI-MS, Compound 1.jpg [Mass spectrum of Compound 1 in methanol]
- 5. FT-IR, Compound 1.pdf [FT-IR spectrum of Compound 1]
- 6. RMN 13C, Compound 1.mnova [RMN 13C spectrum of Compound 1 in CDCl3]
- 7. Assay Biologoly_Compound 1 and 2_Luz vs L. Panamensis_Promastigote.xlsx [Assay Biological of Compound 1 and 2 in the presence of light against L. Panamensis promastigote]
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Optical therapy
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Optical therapy
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I carry out research that is relevant to all aspects of this study.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I carry out research that is relevant to all aspects of this study.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 08 Nov 21 |
read | |
|
Version 2 (revision) 01 Jul 21 |
read | read |
|
Version 1 12 May 21 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)