Keywords
UTI, Prevalence, children under five, antibiogram, Bagamoyo, Tanzania
UTI, Prevalence, children under five, antibiogram, Bagamoyo, Tanzania
Urinary tract infection (UTI) is common in children and tends to recur frequently. UTI is ranked the second most prevalent infection after upper respiratory tract infection in children1,2. The recurrence of UTI is more widespread in girls than in boys3,4. About 30% of children under five suffer from recurrent UTI within the first twelve months after the first occurrence5. If not treated, UTI may lead to pyelonephritis and acute morbidity when associated with abnormalities like vesicoureteral and reflux nephropathy in children. In the long run, UTI may result in parenchymal scarring, hypertension, decreased renal function and renal scarring6,7. For that reason, UTI is a significant contributor to mortality and morbidity in children. However, when recognized and appropriately managed, renal sequelae are rare.
UTI occurs in 2.4-2.8% of children in the United States annually8. The incidence of UTIs is mostly influenced by the host factors such as age and gender. Other risk factors are congenital genitourinary conditions, immature host defenses, lack of circumcision in boys, malnutrition, social status, prior history of UTI, instrumentation, the existence of abnormal urinary tract and the extent of virulence of the etiological agent7,9–11. In both boys and girls, the prevalence of UTI is high in the first twelve months of life and decreases after that5. Shaikh and colleagues reported a UTI prevalence of 7.0% in infants with fever12. In febrile infants aged 0–2 months, UTI prevalence in girls and uncircumcised boys was 5% and 20%, respectively12. In the first six months, the risk of UTI was 10 to 12 higher in uncircumcised boys7. Estimates show that about 7.8% of girls and 1.7% of boys develop UTI by the age of seven. At sixteen years of age, 11.3% of girls and 3.6% of boys may suffer from UTI7.
A prompt diagnosis and appropriate treatment are essential to reduce the morbidity and sequelae following a UTI13. However, diagnosis of UTI in children under two years is usually confounded by the non-specific signs and symptoms of UTI9. Children with uncomplicated UTI may respond to amoxicillin, sulphonamides, trimethoprim-sulfamethoxazole or cephalosporins, concentrating in the lower urinary tract14. Several studies show similar efficacy among the oral and intravenous antibiotics for UTI treatment15. However, studies in high-income countries suggest that UTI causative bacteria increase acquiring resistance to commonly used antibiotics, such as trimethoprim-sulfamethoxazole16,17.
Gram-negative organisms highly contribute to the proportion of uropathogens isolated from children with UTI6,18. Escherichia coli accounts for up to 90% of infections6. Other uropathogens commonly isolated in UTI include Klebsiella pneumoniae, Proteus mirabilis, Citrobacter, Pseudomonas aeruginosa, Enterobacter aerogenes, Enterococcus species and Serratia species18. Proteus mirabilis is more commonly found in boys compared to girls19. Streptococcus agalactiae is commonly isolated from newborns20. Staphylococcus saprophyticus is isolated in sexually active female adolescents and contributes to 15% of UTI cases21.
Malnutrition is a major risk factor for child mortality and adult ill-health. Malnutrition could increase the risk of serious infections22. Malnutrition is still a problem Bagamoyo district in Tanzania due to poor living conditions among people living within and around Bagamoyo23. The living condition may predispose an individual to acquire a UTI. However, the association between malnutrition and the acquisition of UTI has not been studied in this setting. This study was undertaken to determine the prevalence of UTI, antibiotic susceptibility testing of uropathogens and assess the association between UTI acquisition and nutritional status among children under five attending Bagamoyo District Hospital in Tanzania.
This was a cross-sectional study enrolling symptomatic and asymptomatic children under five attending Bagamoyo District Hospital in Tanzania. The inclusion criteria were age range 12 months to 59 months. The study was conducted from April to July 2017. Convenience sampling was used whereby 214 children under five were recruited to participate in the study. The exclusion criteria were children who had recently taken antibiotics, children who were diabetic or HIV positive, and those out of the inclusion age range.
The sample size (n) was calculated according to the formula24 n = z2 * p * (1 - p) / e2, where: z = 1.96 for a confidence level (α) of 95%, p = prevalence and e = margin of error.
According to a study conducted in Tanzania, the prevalence of UTI in children was 16.825, thus making p = 0.168 and taking e = 0.05. The sample size obtained was 214.
Ethical clearance for this study was granted by the Muhimbili University of Health and Allied Sciences Ethics Review Board number 2017-02-20/AEC/Vol XII/59. The parents or guardians permitted their child to participate in the research study following reading and approving the study information provided by the researcher, and parents or guardians signed the consent on behalf of their child. The parents and guardians were informed and consented to the publication of this manuscript.
Mid-stream urine samples were collected from the children attending Bagamoyo District Hospital using the widely recommended urine sampling method in children under five, where guardians were instructed to collect a urine sample in sterile conditions26. Therefore, when the container was one-third complete, the lid was closed and clean catch mid-stream urine samples in sterile containers were transported immediately to the Pharmaceutical Microbiology laboratory for analysis. During transportation, the temperature of 4-8°C was maintained in a cool box containing ice blocks to control microorganism growth27.
A semi-structured questionnaire (see Extended data28), was given to parents or guardians of children under five eligible to participate in this study. The questionnaire collected information about age, gender, type of meal frequently given, and the number of meals per day. We also used the questionnaire to capture height and weight of the children attending the clinic at Bagamoyo District Hospital. Body mass index (BMI) was calculated.
Isolation of bacterial pathogens from urinary samples was carried out using a calibrated loop method in which a sterile standard loop was used to pick 50µL of urine. A loopful urine sample was plated on cysteine lactose electrolyte deficient (CLED) agar. The inoculated plate was incubated at 37°C overnight. The numbers of isolated bacterial colonies were counted as the colony unit to estimate bacterial load/mL of the urine sample. Any sample specimen that contained a bacterial load of ≥105cfu/ml on the calculation of urine samples using a microscope was considered positive for UTI29.
After 24 hours of incubation of the sample on cysteine lactose electrolyte deficient agar (CLED), the growth of bacterial uropathogens in plates was considered positive. The appearance of colonies was observed and recorded. For non-pure bacterial growth, following the use of a sterilized and calibrated loop, a single colony of the pure colony was picked and sub-cultured on MacConkey's agar and incubated at 37°C. After the overnight incubation at 37°C, the bacterial growth appearance, including color and morphology, was observed and recorded30.
Bacteria were identified using the Gram stain test, Kliger's iron agar (KIA), sulfide, indole, motility (SIM) media, catalase and oxidase test31.
The identified uropathogens were subcultured two times before being used for antibiotic susceptibility tests. Antibiotic susceptibility testing was performed as recommended by the Clinical Laboratory Standards Institute guidelines32. The method adopted was Kirby Bauer's discs diffusion assay33. Mueller Hinton agar was used as media for performing antibiotic susceptibility tests.
The antibiotic disc was placed onto the media along the parallel lines separating the standard organism and test organism; seven antibiotic discs were tested against the isolated uropathogens. The seven antibiotic discs tested included amoxicillin-clavulanate acid (20/10µg), ceftriaxone (45µg), ampicillin (25µg), erythromycin (15µg), nalidixic (30µg), trimethoprim-sulfamethoxazole (1.25/23.75µg) and nitrofurantoin (30µg). After overnight incubation, the zone of inhibition of each tested antibiotic disc (6mm disc) was measured using a measuring scale33.
The zone of inhibition's measured diameter was interpreted into resistant, intermediate and sensitive as per the National Committee for Clinical Laboratory Standards (NCCLS)32.
Quality control and review of the collected data were ascertained to remove any errors. Demographic data were available for all participants. For participants who tested negative for urinary tract infection, the corresponding laboratory variables were marked as 'NA; to indicate that data was not applicable in the dataset. The cleaned data was entered into the Statistical Package for Social Scientists (SPSS version 20) computer program and subjected to analysis. The data was analyzed to provide frequency tables. The Pearson chi-square test was employed to determine the association between the demographic data and UTI status, taking a P-value < 0.05 as a significant cutoff at a 95% confidence interval.
A total of 214 urine samples were obtained from children aged between 12 months and 59 months who visited Bagamoyo District Hospital (see Underlying data28), including 123 females and 91 males (Table 1). Demographic data were available for all the 214 participants, while the laboratory analysis was only done for the 35 samples that tested positive for urinary tract infection.
The mean age was 27.1 months. The majority of the children (37.8%) were in the age range 13-24 months, followed by 37.3% in the age range 25-36 months (Table 1).
The prevalence rate of UTI among children attending Bagamoyo District Hospital was 16.4% (Table 2).
| UTI status | N (%) |
|---|---|
| Negative | 174 (83.6) |
| Positive | 35 (16.4) |
| Total | 214 (100) |
The most common causative agent of UTI in children attending Bagamoyo district was E. coli (65.7%), followed by Klebsiella spp (17.1%) (Table 3).
| Organism | N (%) |
|---|---|
| Proteus spp. | 4 (11.4) |
| Escherichia coli | 23(65.7) |
| Pseudomonas spp. | 1 (2.9) |
| Klebsiella spp. | 6 (17.1) |
| Staphylococcus spp. | 1 (2.9) |
| Total | 35 (100.0) |
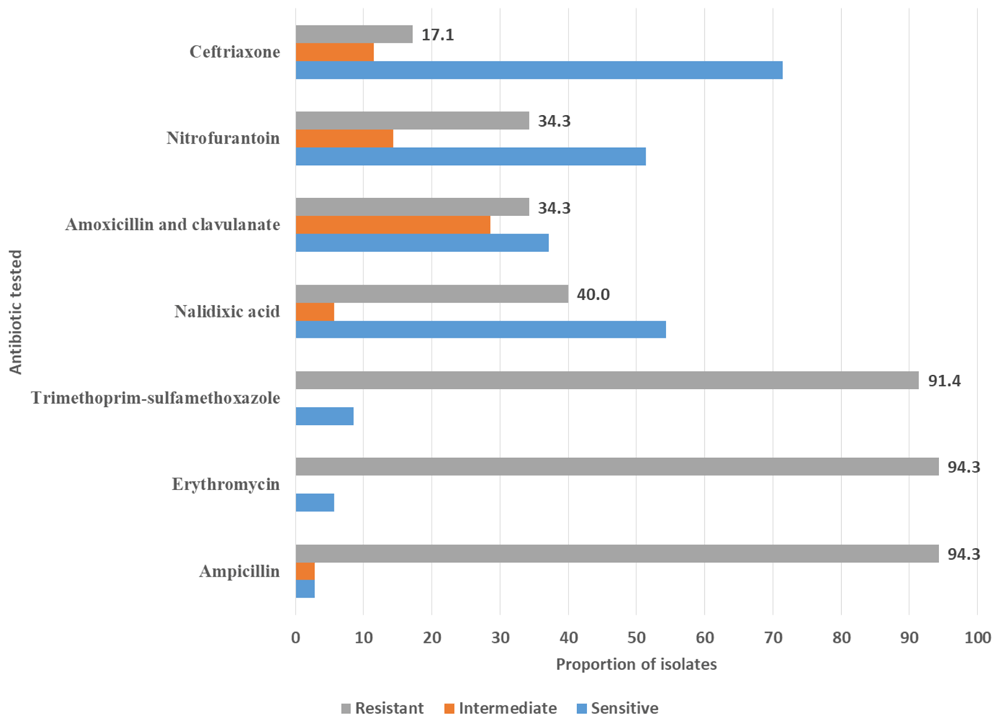
A proportion of 94.3% of the five uropathogens was resistant to ampicillin, followed by erythromycin and trimethoprim-sulfamethoxazole proportion of resistant isolate 94.3 and 91.4%, respectively (Figure 1).
All Proteus species (100%) were resistant to ampicillin and erythromycin (Figure 2), while for E. coli, the proportion of isolates resistant to these two antibiotics was approximately 90%.
Urinary tract infections (UTIs) are common causes of mortality and morbidity in children under five34. In this study, the prevalence of UTI in children attending the clinic at Bagamoyo District Hospital was 16.4%. This is comparable to the 16.8% prevalence reported in a study conducted in Muhimbili National Hospital (MNH) in Tanzania by Francis and colleagues in 201025. Also, in this study, 123 girls and 91 boys were involved. The prevalence of UTIs in girls and boys was 7.9 % and 8.4%, respectively. These results were quite different from previous studies at MNH, which are 18.8% and 15.0% for girls and boys, respectively25. A study conducted in Enugu, Nigeria, found the prevalence of UTI among children under five to be 11%35.
Of all children recruited, 81 (37.8%) were aged between 13 to 24 months and 80 (37.3%) were constituting about two-thirds of the recruited children. The age range 13-24 months had the highest rate (6.5%) of UTI, followed by 25-36 months (4.2%). The rate significantly decreased with increasing age (p-value =0.05). None of the children in the age group 49-59 months tested positive for UTI.
Even though our finding between girls and boys was insignificant, the high prevalence of UTIs in boys than in girls can be explained by the fact that most boys are not yet circumcised at this age. In another study, uncircumcised male infants less than three months of age had the highest baseline prevalence of UTI12. The gender of the child and the parent or guardian's employment status was not associated with UTI positivity.
Similarly, nutritional status (e.g., taking additional meals other than breastfeeding) did not affect the UTI positivity. The mean body mass index for children who were UTI positive was 20.1, which was not statistically different from the negative UTI children with a body mass index of 20.2. In the study in Enugu, Nigeria, females were more likely than males to have UTI positive status35. The lack of association between UTI and nutritional status was also found in another study in rural Africa36. However, a large meta-analysis involving more than 3000 children indicated that the children with malnutrition were more likely to suffer from UTI than the healthy controls37.
The most common causative agent of UTI in children attending Bagamoyo District Hospital was E. coli (65.7%), followed by Klebsiella spp (17.1%), Proteus spp (11.4%), Pseudomonas spp (2.9%) and Staphylococcus spp (2.9%). Similar results were reported by Aiyegoro et al., who reported Escherichia coli (57.8%) in Nigeria38. Similar results were also reported by Bahati et al., who reported that 70% of isolates were Escherichia coli conducted at Bugando Medical Center in Mwanza, Tanzania30. In Turkey, similar results were obtained39, where E. coli was the most prevalent uropathogen with an isolation rate of 64.2%. In Enugu, Nigeria, the organisms isolated from the 22 positive urine cultures were E. coli 31.8%, Staphylococcus aureus 22.7%, Klebsiella species 13.6%, Proteus species 4.55% and Pseudomonas species 4.55%35. In another study in Nigeria, the isolation rate of E. coli was 37%40, indicating higher isolation rates of E. coli in the current study.
In this study, seven antibiotics were used to study the antibiogram of uropathogens isolated from urine samples in children under five, namely amoxicillin-clavulanate, ceftriaxone, ampicillin, erythromycin, nalidixic acid, trimethoprim-sulfamethoxazole and nitrofurantoin. The overall percentage of isolates' resistance was high for ampicillin 93.4%, erythromycin 93.4%, trimethoprim-sulfamethoxazole 91.4%. The resistance was relatively less in nalidixic acid 40%, amoxicillin-clavulanate 34.3%, nitrofurantoin 34.3 and ceftriaxone 17.1%. Therefore, the isolated uropathogens showed high in-vitro resistance to ampicillin, erythromycin, and trimethoprim-sulfamethoxazole. At least 90% of five uropathogens in the 35 positive samples were resistant to these antibiotics. These resistance levels are much higher than reported in a study comprising of 17,164 urine cultures41, where the antimicrobial resistance rates were: ampicillin 36.3%, amoxicillin/clavulanic acid 24.7%, cefuroxime 16.8%, co-trimoxazole 31.1%, ciprofloxacin 14.7%, fosfomycin 14.5%, nitrofurantoin 15.6% and 3rd generation cephalosporins 9–11%41.
On focusing on the antibiogram of all 23 E. coli isolates in this study, the resistance rate to antibiotics was ampicillin 91.3%, erythromycin 91.3%, trimethoprim-sulfamethoxazole, 91.3%, nalidixic acid 39.1%, amoxicillin-clavulanate 34.8%, nitrofurantoin 30.4% and ceftriaxone 17.4%. These findings were comparable to the findings reported by Bahati et al., conducted in Mwanza Tanzania30. In another study conducted in northwestern part of Tanzania, resistance rates of E. coli were ampicillin (98.4%), trimethoprim-sulfamethoxazole (95.3%), amoxicillin-clavulanate (87.5%), cephalexin (61%), cefaclor (43.8%), gentamicin (21.9%), ceftriaxone (14%), nitrofurantoin (12.5%), ciprofloxacin (11.6%), ceftazidime (11%) and cefepime (3.1%)42. The antibiogram of E. coli was similar to that observed in a study in Turkey39.
The prevalence of UTIs for children under the age of five in this study was 16.4%. Escherichia coli was the most common bacteria in UTIs, followed by Klebsiella spp. There was high in-vitro antibacterial resistance to ampicillin, trimethoprim-sulfamethoxazole and erythromycin with Proteus, Pseudomonas and Klebsiella species highly resistant to these three antibiotics. There was no association found between malnutrition status and the UTI.
These findings imply that children under five attending health facilities should be evaluated for UTI. Due to high resistance patterns of erythromycin, ampicillin and trimethoprim-sulfamethoxazole, these agents' routine use for treating UTIs in children under five should be evaluated at the health care facility. These agents need to be used following susceptibility testing results. Therefore, continuous surveillance for antimicrobial stewardship and surveillance is required to curb the increasing resistance patterns of uropathogens to manage UTI and other infections successfully.
One limitation of the study is the study's cross-sectional nature, whose findings may not extrapolate to other regions in Tanzania and abroad. Nevertheless, the study sheds light on the prevalence of urinary infections, the bacteria commonly isolated, and these isolates' antibiotic sensitivity.
Mendeley Data: Dataset for a cross-sectional study on the prevalence of urinary tract infections and antibiogram of uropathogens isolated from under-five children attending Bagamoyo district hospital in Tanzania - dataset. http://dx.doi.org/10.17632/ktzzsfvt79.328.
This project contains the following underlying data:
Mendeley Data: Dataset for a cross-sectional study on the prevalence of urinary tract infections and antibiogram of uropathogens isolated from under-five children attending Bagamoyo district hospital in Tanzania - dataset. http://dx.doi.org/10.17632/ktzzsfvt79.328.
This project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Jun 21 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)