Keywords
metabolic syndrome, green tea, green coffee, combined extracts
metabolic syndrome, green tea, green coffee, combined extracts
Metabolic syndrome is a global public health concern. The prevalence of this disease is estimated to be about one-quarter of the world population. Metabolic syndrome is a condition that consists of risk factors such as abdominal obesity, increase triglycerides (TG), hyperglycemia, hypertension, and low high-density lipoprotein cholesterol (HDL-C) levels. Individuals with metabolic syndrome have a two to fivefold risk of developing diabetes, cardiovascular disease, stroke, and death from all causes1. The pathogenesis of metabolic syndrome involves the strong interaction of genetic and modifiable risk factors that constitute the metabolic syndrome. Previous studies have shown that nuclear factor-kappa B (NF-kB) signalling is a crucial pathway in chronic low-grade inflammation process and has been studied extensively in the context of obesity and the metabolic syndrome2,3. Management of this disease consists of a dual approach that combines lifestyle modification and pharmacological intervention on those risk factors. During the past decades, various natural compound derived from plant extracts showed a beneficial effect in the management of metabolic syndrome4.
Tea, dried leaves of Camellia sinensis, is classified into green, black, and Oolong tea according to its leaf treatment. It contains numerous catechins, mainly epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatechin-3-gallate (EGCG)5,6. In recent years, green tea and its constituents have been massively studied for its beneficial health effects, including alleviation of metabolic syndrome. Most of these studies showed green tea extract and its isolated constituents lowered body weight, blood glucose levels, and increased insulin sensitivity in animal models with metabolic syndrome induced either by high-fat diets, insulin resistance, or genetic modification7. A meta-analysis by Zhong et al.8 also revealed that tea extract reduced body mass index (BMI) and body weight in metabolic syndrome patients. Comparable with green tea, green coffee has recently drawn attention due to its health benefit. Green coffee consists of phenolic compounds, such as chlorogenic acids (CGA), and cinnamic acids (caffeic, ferulic or coumaric acid) which are known as protective agents against metabolic syndrome and type 2 diabetes mellitus9. The meta-analysis by Roshan et al.10 and Nikpayam et al.11 showed green coffee administration had an ameliorating effect on metabolic syndrome parameters, such as systolic blood pressure, fasting blood glucose, and homeostatic model of assessment of insulin resistance (HOMA-IR). In addition, both green tea and green coffee have anti-inflammatory action and has been used as functional food for some metabolic disease protection12,13. However, some studies reported drawbacks of green coffee administration due to its caffeine content14–16. Hence, our study used decaffeinated coffee extracts to avoid the disadvantage of caffeine from coffee intake. Moreover, the CGA antioxidant activity is determined by the coffee’s roasting level17,18. Light roasted coffee beans possessed the highest CGA antioxidant activity. Our previous study showed that light roasted green coffee had a beneficial effect on adiponectin and insulin resistance amelioration in metabolic syndrome rat models19. While many studies reported the beneficial effect of green tea or green coffee extract on several diseases, few reported the impact of the combination of these two compounds. A study by Tulp et al.20 showed that natural compounds have shown greater activity when they are present in a mixture. Considering this, our study aimed to investigate the potential effects of green tea and decaffeinated light roasted green coffee combination in metabolic syndrome rats.
All experimental procedures were approved by the ethical committee of Faculty of Medicine, Brawijaya University with registration number 405/EC/KEP/10/2016. All efforts were made to ameliorate harm to the animals by using the standard protocol from the Indonesian Ministry of Health ethical research guidelines for animal experimental research.
A total of 25 male Sprague Dawley rats aged nine weeks old were obtained from the National Agency of Drug and Food Control, Indonesia. The number of animals was calculated using the Festing formulation21. The study was performed in the Animal Physiology Laboratory, Faculty of Mathematics and Natural Sciences, from March to July 2017. Rats were housed individually in a metabolic cage sized 50 cm x 30 cm x 15 cm at a temperature of 25°C and 40%–70% relative humidity. They were maintained with food and drink ad libitum with 12:12 hour light-dark cycle. After one week of acclimatization, the healthy rats were randomized using Excel (=RAND function) to determine their diet - either normal chow, high fat, or high sucrose (HFHS) diet - for the 17 weeks duration of the experimental protocol. On the 2nd week of the protocol, the HFHS fed rats were induced by a single intraperitoneal injection of streptozotocin (STZ) (bioWORLD cat.41910012-4) (30 mg/ kg B.W.). These procedures were performed in order to get experimental an animal model that represented the features of human metabolic syndrome. The experimental animals were not blinded during this study. The animals were fasted for 8-10 hours prior to blood drawing. The blood (0.5 ml) was obtained from the rats’ tail and then centrifuged with 4500 rpm for 15 minutes to get the serum. The fasting blood glucose, triglyceride, and HDL level were determined by GOD-PAP, GPO, and indirect methods, respectively (BIOLABO cat LP80209 for glucose level determination, cat 80019 for triglyceride level determination, cat 86536 and cat 80106 for HDL level determination). Animals with fasting blood glucose levels over 126 mg/dl, triglyceride level over 150 mg/dl, systolic blood pressure over 140 mmHg, and HDL cholesterol lower than 40 mg/dl were considered to have metabolic syndrome, based on NCEP-ATP III criteria22. Five rats were assigned to normal chow without STZ injection (NC). The metabolic syndrome rats were weight-matched distributed into four experimental groups (five rats/group) (i) HFHS and STZ injection (MS), (ii) HFHS, STZ injection, and green tea extract at 300 mg/kg B.W. (GT), (iii) HFHS, STZ injection, and green coffee extract at 200 mg/kg B.W. (GC), and (iv) HFHS, STZ injection, and combination extract of green tea and green coffee at 300 mg/kg and 200 mg/kg B.W, respectively23. The doses of the extract were obtained from our previous studies19,24,25. The dose of extract was given in millilitres based on weekly measured body weight and given via oral gavage daily. The food and water intake were recorded daily. The sacrifice procedures were explained as follows: (1) The rats were fasted for 12 hours; (2) After that, the rats were anesthetized by diethyl ether prior to the euthanasia in order to prevent painful euthanasia ; (3) After the rats being anesthetized, they were euthanised by cervical decapitation. (4) Immediately after the rats died the blood was drawn from the heart into a microcentrifuge tube; (5) The hepatic tissues were obtained from the right lobe. Serum samples were obtained by centrifugation at 4,000 x g for 15 minutes at 4°C.
The coffee bean was obtained from Dampit coffee plantation, Malang, Indonesia (800 MAMSL). An automatic coffee roaster (N500i) light roasted Coffea canephora var robusta at 180-200°C until the first crack. Furthermore, the coffee bean was mashed with a coffee grinder and then macerated by ethanol 95% to produce the crude extract. The ethanol solvent is used due to its polar feature that could attract the active compounds contained in the green coffee bean. Furthermore, the crude extract was filtered using a filter cloth to separate the liquid phase from the solid phase. Moreover, the liquid phase was concentrated using a rotary evaporator (RV10 autoV, IKA) at ±40°C. Finally, column chromatography completed using silica gel C18 17% (SiliaBond® C18, SiliCycle Inc) as a static phase to reduce the caffeine content, to attract more CGA, and to separate them from other substances, and then the filtered product was evaporated18,26.
The green tea was obtained from Sukawana green tea plantation, Bandung, Indonesia (1550 MAMSL). The second and third uppermost young green tea leaves were extracted. Green tea leaf weighing 500 grams was dried using a cabinet dryer (temperature of 50° C) for 8 hours to obtain green tea with 8 – 10% water content was measured using gravimetric analysis. The green tea was mashed with a blender and then boiled at 80°C for 30 minutes. The crude extract was filtered using a filter cloth to separate the liquid phase from the solid phase. The liquid phase was concentrated using a rotary evaporator at the temperature of ±40°C. Finally, column chromatography completed using silica gel C18 17% (SiliaBond® C18, SiliCycle Inc) as a static phase to attract bioactive compounds and to separate them from other substances, and then the filtered product was evaporated27,28.
For the determination of caffeine and chlorogenic acid levels in the coffee, and epigallocatechin gallat level in the green tea, 1 gram of extract was diluted with 100 mL of distilled water. The HPLC analysis was performed using a Shimadzu Brand chromatograph (model SCL10AVP, Japan) that set up with a C-18 reverse-phase column (Shim-pack VP ODS 5μm 150 x 4,6 mm). The HPLC was paired to a UV/visible spectrophotometric detector (SPD-20-A UV Vis Detector) then connected by an interface (CBM-101) to a microcomputer for data processing. The protocol for analysis were as follows: (1) flow (1 mL/ min for caffeine and chlorogenic acid and 0,45 ml/min for epigallocatechin); (2) mobile phase method using isocratic method with 25% methanol for caffeine and chlorogenic acid and 0,1% TFA in acetonitrile for epigallocatechin; (3) static phase silica C18 with column temperature 25°C; (4) 10 μL of injection volume for caffeine and chlorogenic acid and 1 μL for epigallocatechin ; (5) the wavelength detection at 272 nm for caffein, 324 nm for chlorogenic acid, and 280 nm for epigallocatechin; (6) run time 15 minutes for caffeine, 30 minutes for chlorogenic acid, and 20 minutes for epigallocatechin. The concentrations of the compounds were determined with standard concentration curves.
Decaffeinated light roasted Green coffee extract and green tea extract doses were determined from the previous study in our laboratory. The optimum dose was 300 mg/bw.t for green tea extract and 200 mg/bw.t for green coffee extract19,24,25. Daily food intake and fluid intake was measured every day, and body weight was measured every week. The food and fluid intake for each rat was measured by subtracting the measured amount provided by the remaining amounts in the cage.
The serum concentrations of fasting glucose (BIOLABO, cat no. 80009), triglycerides (TG) (cat no. 80019), and HDL-Cholesterol (BIOLABO, cat no. 86516) were measured enzymatically using commercial kits. C-Reactive Protein (Elabscience cat no. E-EL-R0506) and TNF-a (Elabscience cat no. E-EL-R0019) were analyzed by enzyme-linked immunosorbent assay (ELISA).
Blood pressure was measured using the tail-cuff method with a sphygmomanometer technique (Ugo Basile 58500) at the baseline and the end of the experiment. Three readings are taken consecutively, and the average was then calculated and taken as a final reading for SBP.
Total RNA of liver tissues was isolated using the Easy Blue (Intron Biotechnology, cat no. 17061) according to the manufacturer's protocol. Reverse transcription reaction was performed using a ReverTra Ace-α kit (Toyobo, FSK-101). Then the RNA expression levels were carried out using PCR Light Cycler 96 system (Takara, cat no. TP600) using a GoTaq Green Master PCR Kit (Promega, cat no, M7822) according to the manufacturer's protocols. Primer sequences were as follows: B-actin, forward:5´- TGA GAG GGA AAT CGT GCG TGA CAT-3´ and reverse: 5´-ACC GCT CAT TGC CGA TAG TGA TGA-3´; NF-KB, forward:5´-AAC GCA TCC CAA GGT GCT GGA A-3´ and reverse: 5´- GCA GCT GGA AAA GCT CAA GCC A-3´; TNF-α, forward:5´-CGT CAG CCG ATT TGC CAT TTC-3´ and reverse: 5´-TGG GCT CAT ACC AGG GCT TG-3´; IL-6, forward:5´- CCC AAC TTC CAA TGC TCT CCT AAT-3´ and reverse: 5´-GCA CAC TAG GTT TGC CGA GTA GA-3´. The PCR cycling conditions were as follows: 5 min at 95 °C; 35 cycles of 30 s at 95 °C, 30 s of annealing at 52.7°C, 52.7°C, 54.7°C, and 55 °C for NF-KB, TNF-α, IL-6 respectively, followed by extension for 30 s at 72 °C; and a final extension for 10 min at 72°C. The mRNA levels were quantified using spectrophotometer at 260 nm and 280 nm. of the target genes normalized to the expression level of β-actin.
For assessing TNF-α and CRP secretion, the blood of the rats collected and serum separated by centrifugation. Serum TNF-α and CRP concentrations were measured using an enzyme-linked immunosorbent assay according to the manufacturer's instructions (Elabscience E-EL-R0019 and E-EL-R0022 respectively). The optical density (OD) value was read by ELISA reader machine (Biotek ELx808) and the standard curve was generated to get the formula for TNF-α and CRP quantification. Measurements performed were in two replicates. The results presented are as ng/ml.
The experimental unit was a group of animals and the data obtained was presented in the form of the mean value and standard error of mean (SEM) calculated with GraphPad Prism 8.3.1 software. An independent t-test was used to analyze two of the tested group. Statistically significant differences were defined as p<0.05.
According to HPLC analysis, the concentration of epigallocatechin-3-gallate in our green tea extract was 74,126 µg/g. While the concentration of chlorogenic acid and caffeine, and polyphenols in green coffee, was 27,134 and 43,473 µg/g green coffee extract, respectively29.
Assessment of body weight, systolic blood pressure, fasting blood glucose level, triglyceride and HDL cholesterol plasma level of combination HFHS diet and a low dose of STZ injection revealed the presence of metabolic syndrome symptoms as classified by the NCEP-ATP III criteria. We observed higher body weight, systolic blood pressure, fasting blood glucose level, triglyceride plasma levels and lower plasma levels of HDL cholesterol in rats that received HFHS diet and STZ injection compared to control rats (Figure 1a and b), as expected.
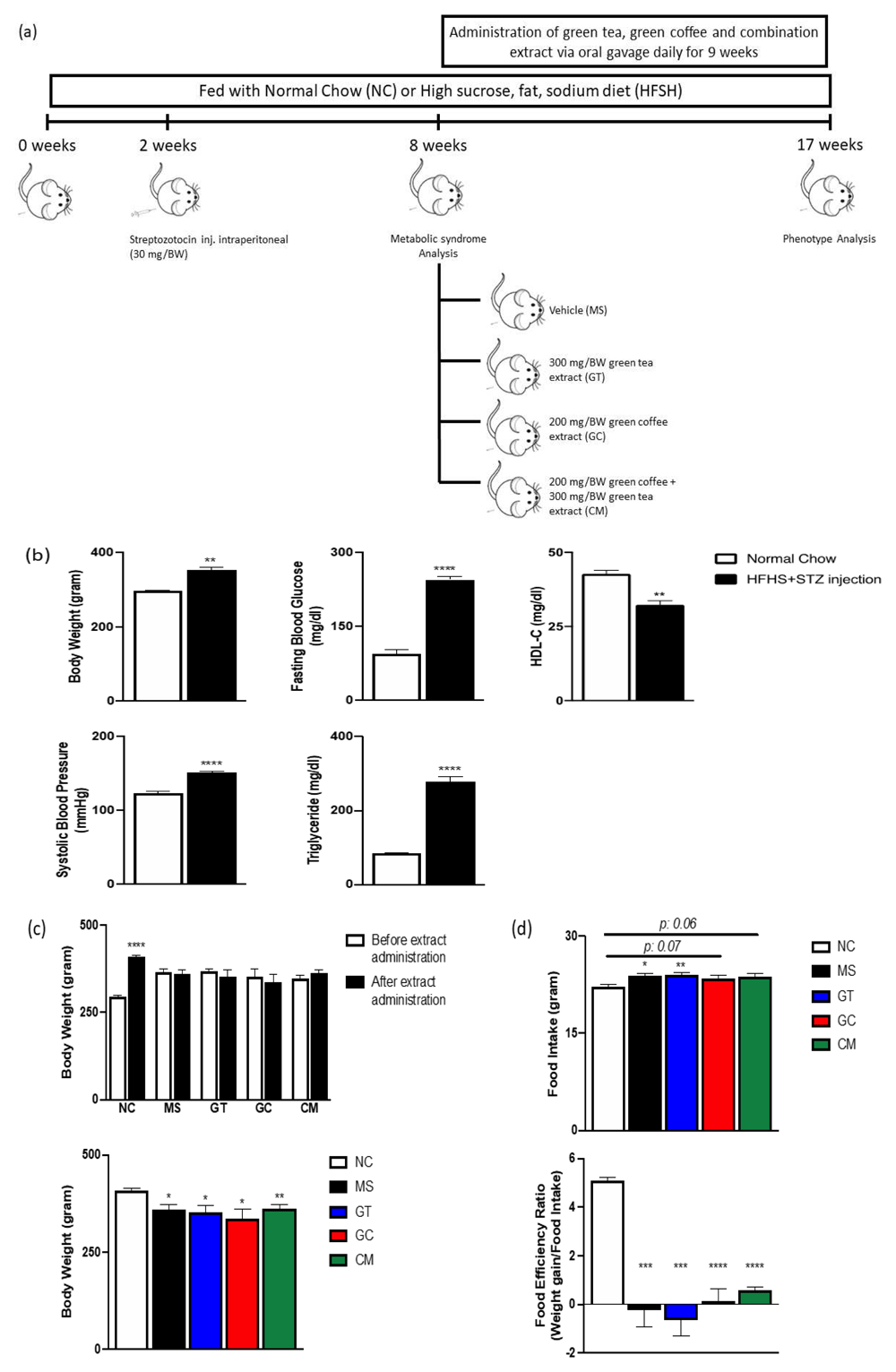
(a) Schematic diagram of study’s experimental design. (b) Body weight, systolic blood pressure, fasting blood glucose, triglyceride level and HDL cholesterol level at 8 weeks. (c) Body weight before extract administration (above) and after 9 weeks administration (above, below). (d) Food intake and food efficiency ratio after 9 weeks intervention. Data are expressed as mean ± SEM (N=4-5). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with NC.
Then, during the nine following weeks, the rats that met NCEP-ATP III criteria were subjected to HFHS alone or HFHS with 300 mg/BW of green tea extract (GT), 200 mg/BW of decaffeinated light roasted green coffee extract (GC), and combination of those extracts (CM) (Figure 1a).
At the end of the intervention, all HFHS-fed- and STZ-injected rats had significantly lower body weight compared to normal chow-fed rats (Figure 1c). Additionally, all metabolic syndrome rats consumed more food (Figure 1d). These results indicate that the food efficiency ratio (weight gain/food intake) of all STZ-treated animals was significantly lower (***p<0.001, ****p<0.0001) compared to that of normal chow-fed rats (Figure 1d).
All supplemented metabolic syndrome rats presented reduced systolic blood pressure (Figure 2a). Green tea and combination of green tea and green coffee could significantly reduce the (*p<0.05) blood pressure after nine weeks of administration; however, the blood pressure of decaffeinated light roasted green coffee only-treated rats was not significantly different compared to their blood pressure before the intervention. Interestingly, compared to the metabolic syndrome rats (MS) group, the green tea extract (GT) group had the lowest systolic blood pressure. Moreover, the green tea treated rats' blood pressure was similar to those in normal chow-fed rats. These results suggest that green tea aloneor could significantly improve the systolic blood pressure of metabolic syndrome rats.
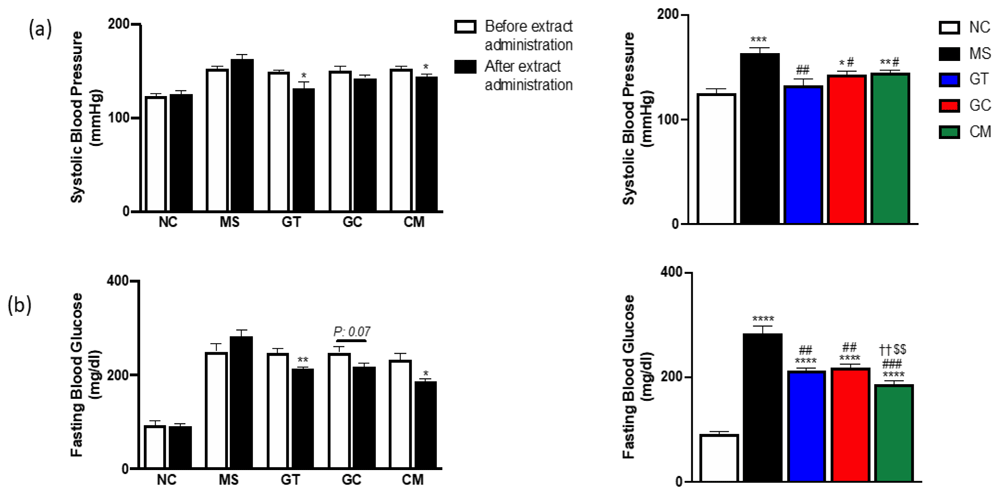
Systolic blood pressure (a) and fasting blood glucose (b) before extract administration (left) and after 9 weeks extract administration (left, right). Data are expressed as mean ± SEM (N=4-5). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with NC. #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 compared with MS. ††P<0.01 compared with GT. $$P<0.01 compared with GC.
All treatments successfully lowered the fasting blood glucose (FBG) levels in rats (Figure 2b). Compared to the blood glucose level before the intervention, only green tea-treated and combination-treated rats demonstrated a significant reduction in FBG. Although the FBG of every treated rat was significantly higher compared to its on normal chow group, combining green tea and green coffee could dramatically lower the fasting blood glucose among the treatment group. In general, green tea and decaffeinated light roasted green coffee combination could improve fasting blood glucose.
Compared to levels before the intervention, all given extracts could significantly reduce triglyceride (TG) levels (*p<0,05) and improve HDL cholesterol levels (HDL-C) (*p<0.05, **p<0.01) (Figure 3a and 3b). After nine weeks of administration, only CM group rats had significantly reduced TG level compared to MS group rats, also, those rats had the lowest TG levels. In contrast, all treated rats had significantly higher HDL-C levels compared to metabolic syndrome rats. These data illustrated that the combined extract group had an improved the lipid profile.
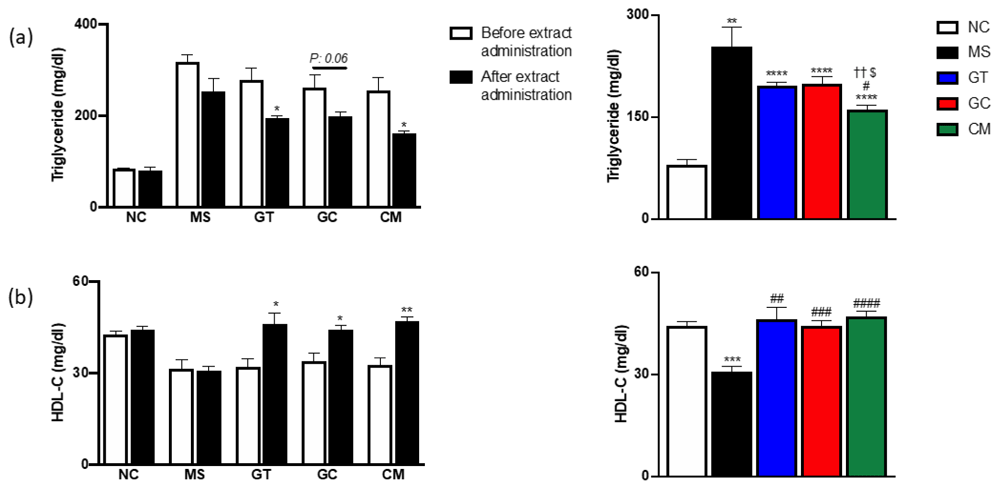
Triglyceride (a) and HDL cholesterol level (b) before extract administration (left) and after 9 weeks extract administration (left, right). Data are expressed as mean ± SEM (N=4-5). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with NC. #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 compared with MS. ††P<0.01 compared with GT. $P<0.05 compared with GC.
The intervention rats showed a reduction of inflammatory markers in plasma and liver tissue compared to MS rats (Figure 4a and 4b). The combined extract-treated rats had the lowest plasma level of TNFα. Moreover, those rats also had the lowest relative mRNA expression of NF-kB, TNFα, and IL-6 in liver tissue, suggesting green tea and decaffeinated light roasted green coffee synergistically reduced inflammatory markers in both plasma and liver tissue
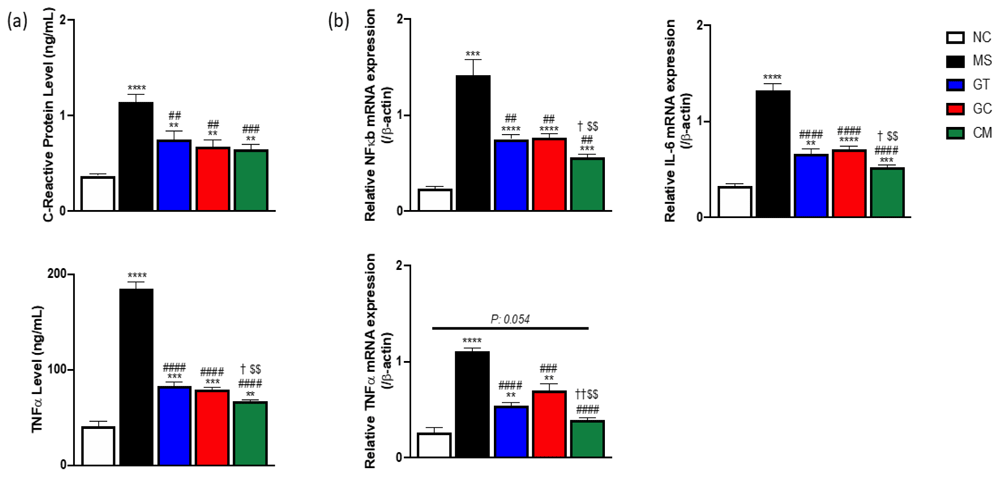
(a) The plasma level of C-reactive protein (CRP) (above) and TNFα level (below). (b) Relative mRNA expression of NF-κB, TNFα, and IL-6 of liver tissue. Data are expressed as mean ± SEM (N=4-5). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with NC. #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 compared with MS. †P<0.05, ††P<0.01 compared with GT. $P<0.05, $$P<0.01 compared with GC.
In agreement with the previous literature30,31, we showed that the administration of green tea extract was sufficient to reduce blood pressure by 19%, which was similar to normal chow group. In contrast to green tea treatment, we did not find any differences in measured blood pressure before and after green coffee extract supplementation alone. Coffee contains many pharmacologically active components that inhibit the anti-hypertensive effect of antioxidative polyphenol, chlorogenic acid, such as hydroxy hydroquinone (HHQ)32. We did not fractionate the green coffee extract to eliminate HHQ, thus, this leads us to hypothesize that HHQ might inhibit the hypotensive effect of green coffee extract. Interestingly, the addition of green tea extract to light roasted green coffee extract was shown to help lower blood pressure compared to that of animals treated with decaffeinated light roasted green coffee extract only, suggesting that green tea extract exerts a more favourable influence on blood pressure.
Compared to metabolic syndrome rats, our study showed either green tea or decaffeinated light roasted green coffee supplementation after nine weeks was sufficient to decrease fasting blood glucose and plasma triglyceride (TG) levels, and these results are supported by previous studies33,34. Unlike the plasma TG levels, findings on the potential effects of tea on HDL-C are mixed. Some studies reported null effects35,36, whereas some showed a significant increase5. However, few investigate the effect of green coffee on plasma HDL-C level. Here, our study presented green tea or green coffee extract alone after nine weeks of administration significantly increased plasma HDL-C level which was similar to normal chow-fed mice. Moreover, we showed that a synergistic effect was not found for the plasma HDL-C levels.
Many studies reported that metabolic syndrome may induce and may be caused by low-grade chronic inflammation that can be detected systematically and within affected tissues, such as adipose tissue, liver, and vasculature37–39. As shown in the present study, both levels of systemic markers of inflammation, including C-reactive protein (CRP) and TNFα, and relative mRNA expression of NF-kB, TNFα, and IL-6 of liver tissue were increased in metabolic syndrome model rats. The liver plays a pivotal role in glucose and lipid homeostasis. Hepatic steatosis and its inflammatory state (steatohepatitis) are closely related to metabolic syndrome40. A recent review has established the critical role of NF-kB on the liver for the development of insulin resistance41. Chronic inflammation activation by NF-kB in the liver activates IKK-β in hepatocytes that exhibit hepatic and systemic insulin resistance, as expected, hepatic overexpression of IkBα, a repressor of NF-kB signalling, reverses the phenotype42.
Recent reviews showed that targeting inflammation in chronic disease may be beneficial in metabolic disorders43,44. While only focusing on inflammation may be harmful to metabolic conditions due to potential immunosuppression, a review suggested anti-inflammatory nutritional intake as a new alternative in modulating metabolism and inflammation45. Several studies have revealed that green tea and green coffee reduced inflammatory markers in animal models12,46–48. Similar to those studies, we demonstrated that green tea or green coffee alone decreased plasma CRP and TNFα levels. Moreover, decreased mRNA expression level of inflammatory genes in the liver was found in rats treated with green tea or green coffee supplementation. Combining those compounds exacerbated the reduction of plasma TNFα and transcription of NF-kB, TNFα, and IL-6. Previous studies have shown that targeting hepatic inflammation by specific deletion of downstream NF-kB signalling, IKK-β and p65, shows an improvement in glucose homeostasis49,50. However, both studies showed that the improvement in insulin sensitivity is limited to the liver. Mice with IKK-β deletion in hepatocytes exhibited liver insulin sensitivity, but insulin resistance in muscle and fat in response to a high-fat diet49. Together these studies conclude that hepatic inflammation contributes to the development of the metabolic disorder, however, further detailed studies are needed to elucidate the beneficial effect of targeting inflammation in the liver.
There are some limitations to this study. First, we did not have data regarding inflammation profiles on other affected tissues, such as adipose tissue, which is directly associated with metabolic syndrome. Second, we could not establish a molecular mechanism for the beneficial effects of combined green tea and green coffee.
In our data highlight that, in metabolic syndrome rats, the combination of green tea and decaffeinated light roasted green coffee ameliorate hepatic inflammatory through suppression of NF-kB, TNFα, and IL-6 gene expression have synergistic metabolic and anti-inflammatory effects.
Figshare: Data for Combination Green Tea and Green Coffee Extract on Inflammatory in Metabolic Syndrome Rat Model. https://doi.org/10.6084/m9.figshare.13249163.v329.
This project contains the following underlying data:
- Data of Metabolic Syndrome Rat Model.xlsx (This file contains the analysed data)
- RAW data metabolic syndrome.xlsx (This file contains the actual observed values of the variables)
- Chlorogenic acid Coffee HPLC.pdf (This file contains the value of coffee chlorogenic acid levels as measured by HPLC)
- Caffeine coffee HPLC.pdf (This file contains the value of coffee caffeine levels as measured by HPLC)
- Green Tea Cathecin HPLC.pdf (This file contains the value of the catechin levels in the tea)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Thanks for Medical faculty of Brawijaya University, Molecular Biology Laboratory of Department of Biology Mathematics and Natural Sciences of Brawijaya University, and Ministry of Research, Technology, and Higher Education of the Republic of Indonesia.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Functional Food, Natural Product, Non-communicable Diseases, Food and Nutrition, Antioxidants, Polyphenols,
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, natural product pharmacology, toxicology, cancer pharmacotherapy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 14 Jun 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)