Keywords
amyloid light chain amyloidosis, bone histomorphometry, bed rest, disuse osteoporosis, immobilization osteoporosis
amyloid light chain amyloidosis, bone histomorphometry, bed rest, disuse osteoporosis, immobilization osteoporosis
Immobilization osteoporosis has been reported to occur after a stroke or spinal cord injury,1-3 spaceflight, and long-term bed rest.4,5 Long-term immobilization or unloading affect both bone formation and bone resorption and lead to bone loss and an increased risk of fracture.6,7 Despite decades of intense research, the effects of long-term bed rest on bone cells and the associated structural changes remain unclear. The effects are difficult to estimate because we cannot exclude factors associated with pre-existing disease and aging. Here, we present a postmortem bone histomorphometric analysis of a man who became bedridden approximately nine months before his death because of severe orthostatic hypotension related to amyloid light-chain (AL) amyloidosis.
An autopsy was performed on a 38-year-old Japanese man with AL amyloidosis who died of interstitial pneumonia. The patient developed AL amyloidosis at the age of 37. At that time, he was otherwise healthy and had no family history of fracture.
At age 37, the patient developed abnormal bowel movements, consisting of alternating constipation and diarrhea, which gradually worsened. He also lost his appetite. He developed orthostatic hypotension and consequently had difficulties standing and sitting and became bedridden. Four months after becoming bedridden, he was admitted to our hospital for diagnosis and treatment.
On admission, the patient was 170.0 cm tall, but his weight had decreased from 90 kg to 62 kg over the previous four months. The laboratory data were as follows: serum albumin, 2.2 g/dL; total protein, 4.2 g/dL; urea nitrogen, 10 mg/dL; serum creatinine, 0.50 mg/dL; calcium, 8.3 mg/dL; phosphate, 3.8 mg/dL; alkaline phosphatase, 151 IU/L Japan Society of Clinical Chemistry (JSCC) method; normal range, 117 to 350), and C-reactive protein, 0.1 mg/dL. An immunologic evaluation found that serum immunoglobulin (Ig) G was 509 mg/dL (normal range, 870-1700 mg/dL); IgA, 98.0 mg/dL (normal range, 110-410 mg/dL); and IgM, 22.0 mg/dL (normal range, 35-220 mg/dL). Serum M-protein was not detectable by immunofixation. Urinalysis detected proteinuria (5.5 g/day), and immunofixation electrophoresis showed kappa (κ)-type Bence-Jones protein in the urine. Kidney biopsy and endoscopic examination of the colon biopsy both showedκ-positive AL-amyloidosis (Figure 1). Examination of the bone marrow revealed 2.8% monoclonal plasma cells (normal value < 10%); however, the patient did not fit the criteria for multiple myeloma because a full skeletal survey did not detect any osteolytic lesions. Thus, primaryκ-type AL amyloidosis was diagnosed.
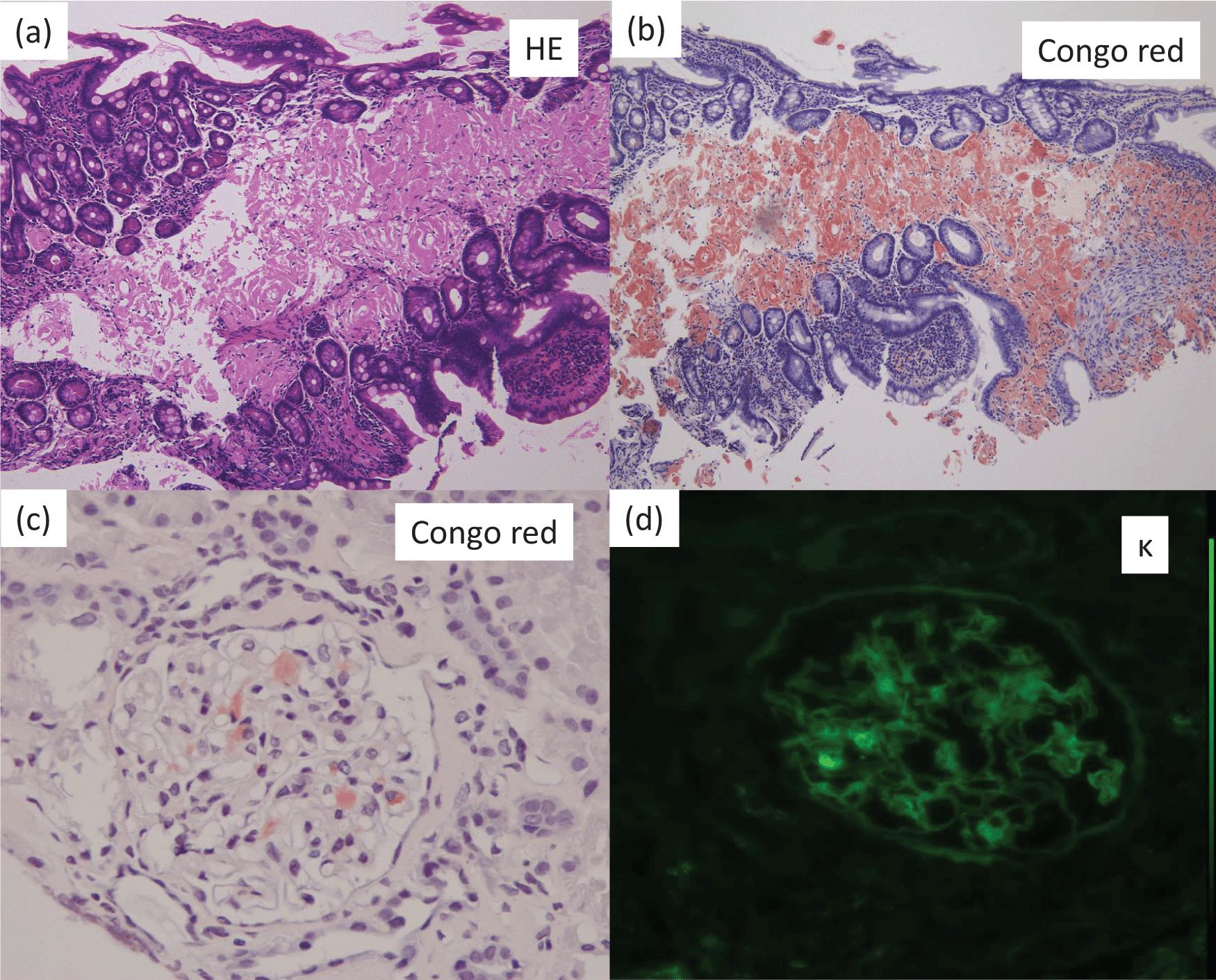
(a): Colon biopsy (PAS stain, light microscopy) (×100). Amorphous material was noted on small arteries and surrounding tissues in the subserosal layer.
(b): Colon biopsy (Congo red stain, light microscopy) (×100). Congo red-material was positive for amorphous material.
(c): Kidney biopsy (Congo red stain, light microscopy) (×400). Congo red material was noted in the glomeruli.
(d): Kidney biopsy (κ-stain, immunofluorescence microscopy) (×400). κ-stain was positive for Congo red material).
At admission, the patient’s blood pressure was 100/60 mmHg in the sitting position but decreased to 60/30 mmHg in the standing position. Therefore, the patient remained in bed. Treatment was started with vincristine (0.4 mg/day for 4 days), adriamycin (14 mg/day for 4 days), and dexamethasone (40 mg/day for 12 days). However, five months after hospitalization, the patient suddenly died of interstitial pneumonia. At death, the patient had been bedridden for nine months.
After obtaining consent from the patient’s family, we performed an autopsy. Bone histomorphometric analysis of the right iliac bone was performed at the Ito Bone Science Institute (Niigata, Japan); tetracycline double labeling was not performed.
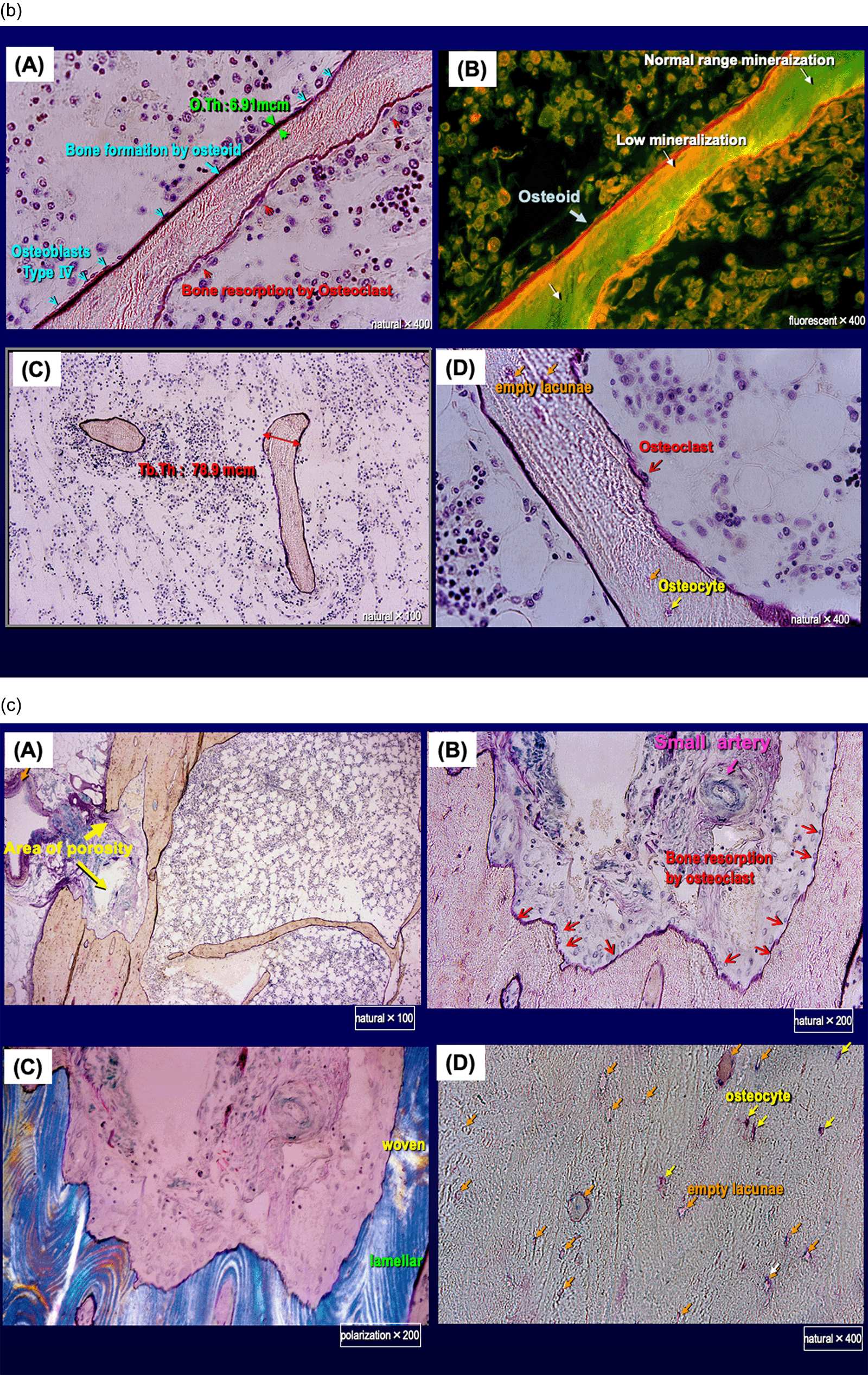
Cancellous bone was assessed by bone histomorphometry (Table 1). All bone volume markers were decreased compared with the age-matched reference ranges presented in the report by Reccker et al.,8 as follows: trabecular bone volume to total volume, 6.77%; trabecular thickness, 78.9 μm; trabecular unit wall thickness, 21.0 μm.
Cancellous bone was assessed by bone histomorphometry. All bone volume markers were decreased compared with the age-matched reference ranges. All bone osteoid markers were also decreased compared with the age-matched reference range. The fibrous tissue volume to total volume could not be assessed, and the eroded surface to bone surface was increased to 16.2%.
All bone osteoid markers were also decreased compared with the age-matched reference range, as follows: osteoid volume to total volume ratio, 0.03%; osteoid volume to bone volume ratio, 0.51%; osteoid surface to bone surface ratio, 4.12%; and osteoid thickness, 6.91 μm. The fibrous tissue volume to total volume could not be assessed, and the eroded surface to bone surface was increased to 16.2%.
The cancellous bone volume was greatly decreased, and trabecular thinning was apparent; the trabecula was preserved, but the number of trabecular termini was increased to 52 points and the number of nodes (bifurcated trabecula) was decreased to 12 points (Figure 2a). Trabecula thinning indicated resorption by osteoclasts, and island bones and clubbing trabecula with bilateral termini were noted. We found more empty lacunae, which are characterized by the disappearance of osteocytes, than lacunae containing osteocytes (Figure 2b).
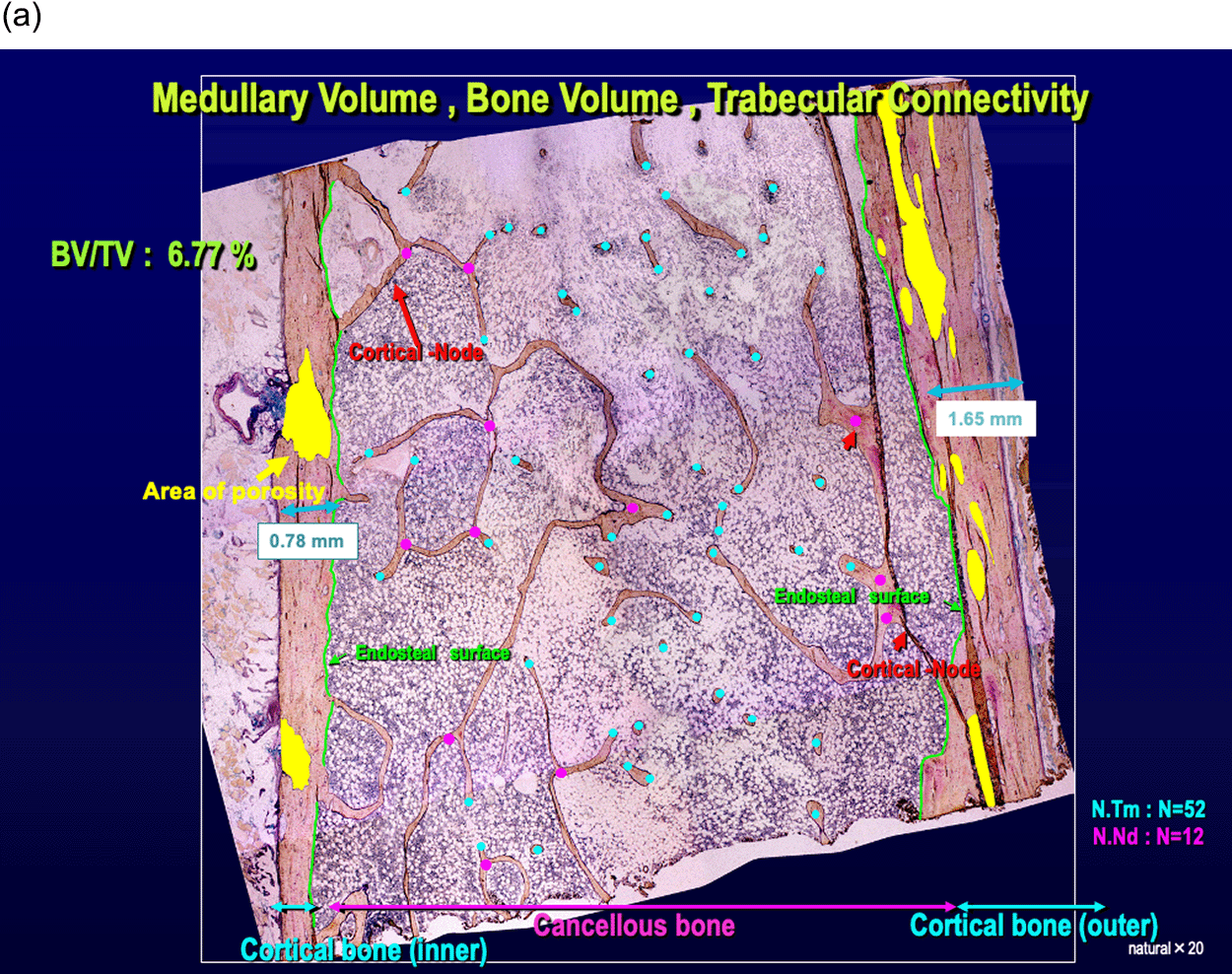
(a) Low-power field of light microscopy (×20).
The cancellous bone volume was greatly decreased, and trabecular thinning was apparent; however, the trabecula was preserved, although the number of trabecular termini (N.Tm; red) was increased to 52 points and the number of nodes (bifurcated trabecula) (N.Nd; blue) was decreased to 12 points. Compared with the width of cancellous bone, the width of cortical bone was preserved with 1.65 mm and 0.78 mm. The number of cortical node structures connecting from the endocortical surface to the cancellous trabecula was decreased.
(b) (A and B): Trabecula thinning indicated resorption by osteoclasts.
(C) Island bones and clubbing trabecula with bilateral termini were noted.
(D) More empty lacunae, characterized by the disappearance of osteocytes, were seen than lacunae containing osteocytes.
(c) High-power field of cortical bone (×100, 200 or 400).
(A, B and C) Large pores associated with enlargement of the bone marrow cavity due to resorption by osteoclasts.
(D) More empty lacunae were seen than lacunae containing osteocytes.
Cortical bone was preserved with cortical bone width of 1.65 mm and 0.78 mm. However, the area of porosity to total cortical bone area ratio, which increases when large pores appear because of enlargement of the bone marrow cavity due to resorption by osteoclasts, was increased to 37.19% and 22.11% (Figure 2a, Table 1). The number of cortical node structure connecting from the endocortical surface to the cancellous trabecula was decreased (Figures 2a and 2c). More empty lacunae were seen than lacunae containing osteocytes (Figure 2c).
Severe osteoporosis of cancellous bone closely associated with immobilization was diagnosed.
Postmortem Iliac bone of a 46-year-old healthy man.
The standard values of cortical bone have not been reported. Therefore, to put the degree of osteoporosis in our patient into context, we determined the parameters of postmortem iliac bone of a 46-year-old healthy man. The control individual showed abundant cortical bone surrounding the cancellous bone. The cortical bone width was 1.45 mm and 0.67 mm, and the area of porosity area to total cortical bone area ratio was 3.2% and 8.45%.
Immobilization or skeletal unloading, which can occur during spaceflight, hindlimb suspension, and long-term bedrest, are well recognized as causes of loss of bone mass and strength. Both clinical and animal studies have reported on the mechanisms of bone loss in these situations.9-16 For example, previous research has examined the effects of tail suspension, tenotomy, or sciatic neurectomy in animal models and of bed rest with a head-down tilt in human studies.9,11,13-15
In animal models, unloading reduces the rate of bone formation because of changes in osteoblast progenitor cell recruitment and defective functioning of differentiated osteoblasts.9-11 On the other hand, the effect of immobilization or skeletal unloading has been reported to cause inconsistent changes in osteoclast surface and number.9-12
In a human study, 12 weeks of bed rest led to suppression of the osteoblastic surface in cancellous bone and an increase of bone resorption and eroded surface in both cancellous and cortical bone, although cortical and cancellous bone volume, as measured histomorphometrically, did not change.7 This study and another one found that mean cortical and trabecular thickness also did not change after long-term bed rest.7,13 Another study found that 120 days of bed rest led to loss of cancellous bone volume.14 However, mean trabecular separation and the node to terminus ratio did not change. The differences in the results of these studies were probably related to differences in their duration, the causes of immobilization, and the age and sex of the participants.15,16
In conclusion, we performed postmortem bone histomorphometric analysis of the iliac bone on a 39-year-old man who had been bedridden for nine months before his death because of severe orthostatic hypotension and severe malnutrition related to AL-amyloidosis. The cancellous bone volume was greatly decreased and trabecular thinning was apparent, although the trabecula was preserved. Cortical bone volume was also preserved. Our findings indicate that immobilization-related osteoporosis is associated mainly with bone loss in cancellous bone.
The significance of this report is limited to the presentation of pathological data showing that the long-term immobilization in the patient with AL amyloidosis leads to severe osteoporosis. However, the patient’s underlying low nutrient condition, which was caused by AL amyloidosis, may have contributed to the bone abnormalities.
This investigation was conducted in accordance with the Declaration of Helsinki. Before his death, the patient provided written informed consent for publication of this case report.
After his death, the patient’s wife provided written informed consent for publication of this case report and any accompanying images.
All data underlying the results are available as part of the article and no additional source data are required.
We wish to thank Mrs. Akemi Ito (Ito Bone Science Institute, Niigata, Japan) for performing the bone histomorphometric analyses.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Human bone biology
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Jun 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)