Keywords
Hyperbaric oxygen therapy; Traumatic brain injury; Post-concussion syndrome; Rivermead Post-Concussion Symptoms Questionnaire; Matrix metallopeptidase 9; Randomized controlled trial.
Hyperbaric oxygen therapy; Traumatic brain injury; Post-concussion syndrome; Rivermead Post-Concussion Symptoms Questionnaire; Matrix metallopeptidase 9; Randomized controlled trial.
Head injury is the leading cause of death and disability among young adults.1 In Indonesia, head injuries account for almost half of all deaths caused by trauma.2–4 The symptoms of head injury range from mild to severe depending on the extent of brain damage.5 Patients with head injuries often experience considerable cognitive, behavioral, and communication impairment.6 These patients deserve prompt, effective treatments that are not only lifesaving but also preserve their brain function.
Hyperbaric oxygen therapy (HBOT) involves the inhalation of 100% oxygen at a higher pressure than the atmospheric standard. Patients inhale 100% oxygen, and pressure increases gradually to 2-3 absolute atmosphere (ATA).7–10 HBOT has become popular in the field of neurology because it inhibits apoptosis, suppresses inflammation, and protects the integrity of the blood-brain barrier (BBB), in addition to stimulating angiogenesis and neurogenesis. The neuroprotective effects of HBOT are most effective during the acute phase, which is the first 24 hours post-head injury.11
The anti-neuroinflammatory properties of HBOT are at least partially exerted by suppressing matrix metallopeptidase 9 (MMP-9) expression.12 MMP-9 is a Zn-dependent endopeptidase enzyme that maintains and remodels the extracellular matrix (ECM). MMP-9 is produced by microglia, neurons, oligodendrocytes, astrocytes, and the vascular endothelium.13 In chronic brain injury, HBOT increases cerebral blood flow, improves any related neuropsychological disorders, and promotes neurophysiological and electrophysiological recovery.14 As such, this therapy improves the quality of life in patients with post-concussion syndrome and prevents its progression to more advanced stages.15 Taken together, these data suggest that HBOT represents a promising therapeutic modality for various forms of head injury.
The persistence of symptoms associated with mild traumatic brain injury (TBI) is referred to as post-concussion syndrome. The majority of patients with post-concussion syndrome recover within three to six months.16 In most clinical trials on the survey-based, patient progress was evaluated by neuropsychological examination such as the Rivermead Post-Concussion Symptoms Questionnaire (RPQ). The RPQ is one of the most commonly used instruments for determining the severity of the symptoms caused by mild to moderate TBI. Individual item scores reflect the presence and severity of post-concussion symptoms that overlap with a wide range of conditions (e.g., pain, fatigue, and mental health conditions including depression). The RPQ is divided into two groups; RPQ-3 and RPQ-13. RPQ-3 consists of three initial symptoms, such as headaches, feelings of dizziness, nausea and/or vomiting. The RPQ-13 is a progression of these initial symptoms, such as noise sensitivity, sleep disturbance, fatigue, feeling irritable, feeling depressed or impatient, forgetfulness, poor concentration, taking longer to think, blurred vision, light sensitivity, double vision, and restlessness.17
Questionnaires are repeatedly administered to monitor the patient's progress over time and promptly identify changes in the severity of symptoms. The recovery process usually requires three to six months of conservative treatment to resolve the symptoms.18
However, these conservative treatments are slow and sometimes ineffective, leaving patients with life-long symptoms ranging from headaches to impaired cognitive function. Therefore, the present study aims to determine whether and how HBOT accelerates post-concussion syndrome recovery by analyzing RPQ scores and MMP-9 levels, respectively. We hypothesize that HBOT can improve post-concussion symptoms by decreasing MMP-9 levels in patients with TBI undergoing HBOT compared with traditional therapeutic approaches.
This study used a randomized controlled trial design to assess levels of MMP-9 and Rivermead Post-Concussion Symptoms Questionnaire (RPQ) scores at pre-treatment (baseline) and post-treatment at weeks one, three, and five.
The study was conducted after obtaining approval from the research ethics committee of Prof. Dr. R. D. Kandou Hospital, Manado (Code number: 125/EC/KEPK-KANDOU/XII/2020). This study was registered with the Research Registry with a registration number no. 6465 on January 17, 2021. Written informed consent was obtained from all the participants. The work was carried out in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines.19,20
This study included 20 patients with mild TBI. The patients were randomly divided according to treatment into a control group and an experimental group. The experimental group received HBOT in addition to the Advanced Trauma Life Support (ATLS) protocol while the control group only received the latter. In accordance with randomized controlled trial design, the patients in the control group were so selected because they refused to receive HBOT. The study was carried out at Dr. R. D. Kandou Hospital, Manado, North Sulawesi, Indonesia.
All patients received standard mild TBI treatment according to the ATLS protocol including head CT scans using computed tomography SOMATOM Scope (Siemens Healthineers AG, Erlangen, Germany) to identify any brain abnormalities. The HBOT group also received 60-minute HBOT sessions, breathing in 100% oxygen at 2-3 ATA, three times during this study at weeks one, three, and five.21
Items in the questionnaires are divided into two groups. The first group (RPQ-3) consists of three items and the second group (RPQ-13) consists of 13 subsequent items.17,18,22 The total score for the RPQ-3 items range from 0-12 and reflect the baseline symptom pool of post-concussion symptoms. A higher RPQ-3 score requires reassessment and close monitoring.23
Prior to initiating treatment in both groups, the RPQ was administered, blood samples were collected for serum MMP-9 assays, and head CT scans were performed to identify any brain abnormalities such as subarachnoid hemorrhage (SAH), epidural hematoma (EDH), subdural hemorrhage (SDH), and intracerebral hemorrhage (ICH). MMP-9 levels were measured in the Biomolecular and Immunology Laboratory of the Faculty of Medicine at Sam Ratulangi University in Manado, Indonesia after every HBOT session for both the HBOT and control groups. At the end of week 1, 3, and 5, all patients were reevaluated for serum MMP-9 levels and RPQ scores. An enzyme-linked immunosorbent assay (ELISA) was used to quantify MMP-9 levels in ng/mL, as described in the MMP9 Human ELISA Kit protocol (Invitrogen Corporation, Carlsbad, CA, USA), catalog number BMS2016-2.9,21,24
Microsoft Excel was used for data entry and R Statistical Software version 3.6.325 was used for all statistical analyses. For the categorical variables, frequency tables were used to assess distributions. Both center and dispersion values were calculated according to the type of variable as was the normality of the distribution for numeric variables. Normally distributed numeric variables are presented as means and standard deviations (SD). If the distribution is abnormal, median values and interquartile ranges (IQR) are given. Differences for each variable according to the treatment group (HBOT vs control) were analyzed using the t-test for numerical variables and the chi-square or Fisher's exact test for categorical variables. Changes in serum MMP-9 levels in the treatment group according to the time of examination were visually evaluated using graphs and linear mixed model analysis with random intercept; these analysis measurements were repeated for each study subject. The effects of HBOT on serum MMP-9 levels and RPQ scores were evaluated using linear regression models. The modeling results are reported as changes in the outcome value for each unit increase in the independent variable, lower and upper limits of the 95% confidence interval (CI), and p-values, which were considered statistically significant below 0.05.
Patient characteristics including age, sex, and the presence of intracranial bleeding are presented in Table 1. The average patient age was 39 years and this was not significantly different between the HBOT and control groups. The male-to-female ratio was approximately 6.5: 3.5. Intracranial bleeding was found in over half of the cases and these were equally distributed between the HBOT and control groups. No patient dropped out during the 6-week study period.
| Characteristics | Total (N = 20) | Control (n = 10) | HBOT (n = 10) | Pa |
|---|---|---|---|---|
| Age (μ ± SD) | 38.9 ± 15.1 | 38.0 ± 15.0 | 39.7 ± 16.0 | 0.809 |
| Sex | ||||
| Male | 13 (65) | 7 (30) | 6 (60) | 1.000 |
| Female | 7 (35) | 3 (30) | 4 (40) | |
| Intracranial bleeding | ||||
| Negative | 6 (30) | 3 (30) | 3 (30) | 1.000 |
| Positive | ||||
| SAH | 5 (25) | 2 (20) | 3 (30) | |
| EDH | 3 (15) | 1 (10) | 2 (20) | |
| SDH | 1 (5) | 1 (10) | 0 (0) | |
| ICH | 5 (25) | 3 (30) | 2 (20) |
Table 2 displays the MMP-9 levels and RPQ scores at different measurement time points. The two patient groups had different MMP-9 serum levels even before treatment was initiated; however, this difference was not statistically significant. While both groups experienced decreases in MMP-9 levels during the course of this study, the reduction in the HBOT group was significantly greater than in the control group (40.6 ng/mL vs 21.7 ng/mL; p < 0.001). Therefore, even though the control group had higher MMP-9 levels at baseline, this was compensated for by the highly significant endpoint difference (the difference between both scores (delta) after five weeks).
| Variableb | Total (N = 20) | Control (n = 10) | HBOT (n = 10) | pa |
|---|---|---|---|---|
| MMP-9 (ng/mL) | ||||
| Baseline | 100.6 ± 17.4 | 113.1 ± 16.2 | 88.2 ± 5.9 | < 0.001 |
| Week 1 | 91 ± 17.9 | 105 ± 13 | 77.1 ± 8.8 | < 0.001 |
| Week 3 | 78.2 ± 21.1 | 96.6 ± 11.5 | 59.8 ± 7.4 | < 0.001 |
| Week 5 | 69.5 ± 24.2 | 91.3 ± 10.2 | 47.6 ± 8.3 | < 0.001 |
| Delta | -31.2 ± 13.5 | -21.7 ± 8.5 | -40.6 ± 10.7 | < 0.001 |
| RPQ Score | ||||
| Baseline | ||||
| RPQ-3 | 10.9 ± 0.9 | 10.7 ± 0.9 | 11.1 ± 0.9 | 0.340 |
| RPQ-13 | 37.2 ± 3.1 | 36.8 ± 2.8 | 37.6 ± 3.4 | 0.574 |
| Week 5 | ||||
| RPQ-3 | 4.8 ± 1.9 | 6.5 ± 1 | 3.1 ± 0.3 | < 0.001 |
| RPQ-13 | 21.9 ± 8.1 | 29.6 ± 2.4 | 14.2 ± 1.2 | < 0.001 |
| Delta | ||||
| RPQ-3 | -6.1 ± 2.2 | -4.2 ± 1 | -8.0 ± 1.1 | < 0.001 |
| RPQ-13 | -15.3 ± 9 | -7.2 ± 3.5 | -23.4 ± 3.7 | < 0.001 |
RPQ scores did not differ between the two study groups at baseline. However, by the end of week five, patients receiving HBOT had marked improvements in their RPQ scores compared with controls (mean 3.1 vs 6.5 for RPQ-3, 14.2 vs 29.6 for RPQ-13; both p-values < 0.001). As a result, the delta scores from baseline to week five in the HBOT group were higher compared with the control group (-8.0 vs -4.2 for RPQ-3, -23.4 vs -7.2 for RPQ-13; both p-values < 0.001).
Figure 1 illustrates the changes in serum MMP-9 levels in both groups at four different time points. MMP-9 levels in the HBOT group are consistently lower and decline notably after two weeks compared with the control group. Figure 2 shows that the patients receiving HBOT had overall lower MMP-9 levels than those in the control group. Similarly, Figure 3 depicts the declining MMP-9 concentration in both groups over time and highlights that HBOT causes MMP-9 values to plummet within four weeks of initiating post-injury treatment compared with controls.
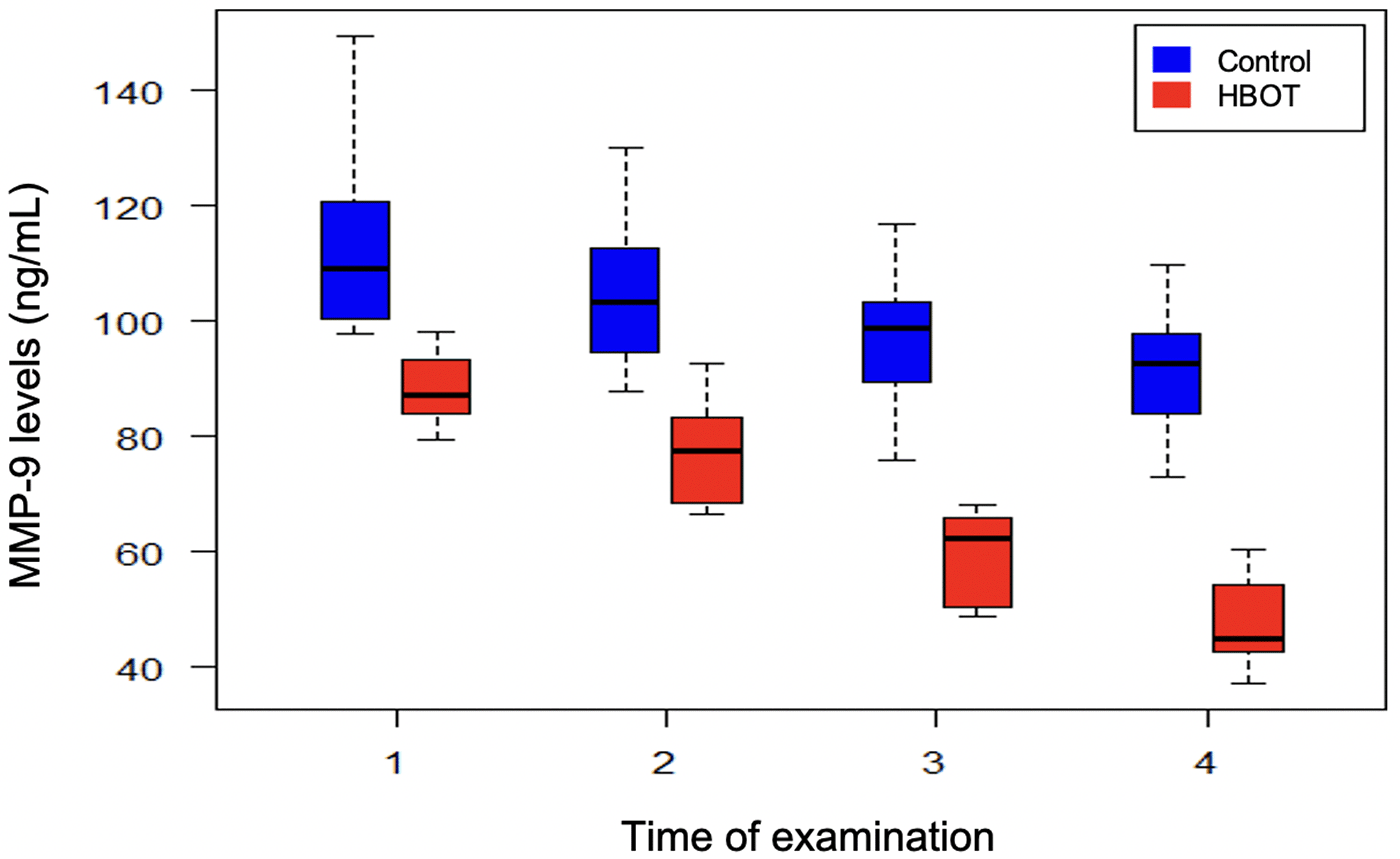
Regression analysis using a linear mixed model revealed that changes in MMP-9 level depended on the interaction between both groups (HBOT vs control) and time (Figure 4). The p-values for the group, time, and the interaction of both variables were highly statistically significant (p < 0.001). There was a decrease in MMP-9 concentration in both groups over time, however, this decline was accelerated in the HBOT group compared with controls.
Table 3 presents the regression analysis of the relationship between the outcome variables (MMP-9 concentration and RPQ scores) and HBOT administration. Scores for the individual variables were marked as “delta” and represent the difference between the results at week five and baseline. Compared with controls, the HBOT group exhibited significant declines in MMP-9 concentration (127.91 vs -9.76; p < 0.001), RPQ-3 (-4.78 vs -2.82; p < 0.001) and RPQ-13 scores (-19.62 vs 12.78; p < 0.001).
Based on their timing and different distinctive underlying pathomechanisms, head traumas are categorized as primary or secondary brain injury.26 Secondary brain injury occurs in the weeks following, and in response to, the primary brain injury, which occurs at the time of and is directly caused by the trauma itself. Patients with secondary brain injury experience biochemical, metabolic, and cellular changes that are orchestrated by a complex biochemical cascade that causes increased intracranial pressure, BBB damage, neuroinflammation, brain edema, brain hypoxia, ischemia, and neurodegeneration.27 The outcome of TBI is dependent on the secondary brain injury process.28 The starting point of our study is based on this secondary pathomechanism and its reversible, dynamic nature.
The ECM and BBB both play important roles in neuroplasticity.29 An imperative factor in the damage of the BBB is MMP-9, which is produced by microglia, the first line of defense against brain injury. As the sensors and effectors of the brain’s immune system, microglial activity is the primary marker of neuroinflammation.30,31
Enzymes such as occludin and claudin cause the basal degradation of endothelial lamina by targeting laminin, fibronectin, collagen, proteoglycan, and tight junction proteins (degradation of ZO-1), which are also the two main functional elements of the BBB.32 The downstream outcomes of the enzymatic digestion of these structural proteins include cytoskeletal damage, the disruption of cellular homeostasis, ischemia, inflammation, tissue necrosis, and apoptosis.33
The stability of the microenvironment surrounding neurons, including the ECM, is necessary for healthy brain function. This stability is sustained by the BBB to maintain brain homeostasis and prevent cell death and dysfunction.32 The integrity of the BBB is the key to the restoration of brain homeostasis after physical injury. The BBB plays a pertinent role in this process because its integrity is influenced by MMP-9 activity, a major effector in secondary brain injury. Therefore, MMP-9 represents a reliable, relevant biological marker for predicting TBI outcome.34
In the present study, HBOT was administered to prevent or inhibit MMP-9 production. HBOT acts on the neutrophil adhesion mechanism in endothelial cells. More specifically, HBOT reduces the expression of endothelial adhesion molecules and inhibits neutrophil adhesion molecule clustering, thus reducing the number of neutrophils adhered to endothelial cells. Decreasing the number of neutrophils activates pro-inflammatory processes including the release of pro-inflammatory cytokines (e.g., IL1B, IL-6, IL8, and TNFa) and decreases the expression of MMP-9.35
The results of this study demonstrate HBOT’s ability to inhibit the production of MMP-9. Similarly, a study in Canada found improved cognitive function, quality of life, and elevated brain activity in patients with mild TBI and prolonged post-concussion syndrome after 40 HBOT sessions over two months.36
In another study, 40 rats were subjected to dynamic cortical deformation (DCD) and then divided into three treatment groups three hours after the initial trauma. 20 rats received HBOT with 100% oxygen at 2.8 ATA for 45 minutes, 10 received 100% oxygen for 45 minutes under normobaric conditions (1 ATA), and 10 were untreated (controls). Neuroinflammatory markers (TIMP-1 and TIMP-2) and MMP-9 levels were measured 96 hours after treatment. MMP-9 levels were found to be significantly lower in the HBOT group relative to controls. Inflammatory infiltration around the focus of necrotic brain tissue was prominent in most untreated animals and was composed predominantly of myeloperoxidase-positive neutrophils. Both normobaric and hyperbaric hyperoxia resulted in a significant decrease of neutrophil infiltration (GLM ANOVA main effect of treatment group: P < 0.0001; This reduction in the neuroinflammatory response was more extended with HBOT in comparison with normobaric hyperoxia (Tukey-Kramer P < 0.05).12
HBOT decreases intracranial pressure (ICP), reduces cerebrospinal fluid (CSF) pressure in patients with acute brain injury, restores the metabolic activity of the substance grisea in a single-photon emission computerized tomography scans of the closed head injury, and to restore glucose metabolism after brain injury.37,38 HBOT decreases mortality rates and improves the functional outcome of patients with severe head injuries. In such chronic brain injuries, HBOT increases cerebral blood flow, improves neuropsychological disorders, and promotes neurophysiological and electrophysiological recovery.39
Several studies have reported that multiple HBOT sessions can improve neurological deficits and cognitive impairment in both acute and advanced chronic phases of head injury in rats.40–42 The long-term therapeutic effects of HBOT are derived from the induction of angiogenesis, neuroplasticity, and proliferation and differentiation of nerve stem cells. When HBOT was administered within three hours post-injury in a fluid percussion mouse model of TBI, there was a significant increase in the number of endothelial cells, neurons, and new glial cells four days after the initial injury.40 Ten daily HBOT sessions at 2.5 ATA for 60 minutes enhances neuroplasticity by increasing axonal sprouting and synapse remodeling, contributing to the restoration of locomotor function in rats with TBI.42 Harch et al. study on the blood vessel density was measured bilaterally in the hippocampus using diaminobenzidine staining and correlated with MWT performance (Morris Water Task). They found increased vascular density in the bruised hippocampus and improved cognitive function in these rats after consecutive HBOT sessions (7 days/week, 1.5 ATA, 90-minute sessions/HBOT) over 40 days.43 Several signaling pathways and transcription factors have been implicated in HBOT-induced neurogenesis including, wingless-related integration site, hypoxia-inducible factors, and cAMP response element-binding.44
The outcome of this study was evaluated by the RPQ. RPQ-13 scores range from zero to 52, with higher scores reflecting more severe post-concussion syndrome. The RPQ-13 items encompass a group of advanced symptoms while the RPQ-3 symptoms include headaches, dizziness, and nausea. All those symptoms included in RPQ highly impact patient participation in social activities, psychosocial functioning, and lifestyle.17 During the three to six months typically required to resolve these symptoms patients are advised to gradually resume their routine activities. If symptoms do not resolve within this period, patients are usually often referred to a specialist for further assessment and treatment services.45
The data collected in the present study showed a notable decrease in the RPQ-3 and RPQ-13 scores. There was no significant difference in the baseline RPQs of the groups at the beginning of the study. However, by the end of the five weeks of treatment, the patients receiving HBOT had much better RPQ scores (mean 3.1 vs 6.5 for RPQ-3, and 14.2 vs 29.6 for RPQ-13; both p-values < 0.001). As a result, the delta score from baseline to the end of week five in the HBOT group was significantly lower than the control group [-8.0 vs -4.2 for RPQ-3 (p < 0.001), -23.4 vs -7.2 for RPQ-13 (p < 0.001)].
The decline in RPQ scores over time was significant. RPQ-3 (-3.80 (p < 0.001)) and RPQ-13 (16.20 (p < 0.001)). HBOT administration in patients with mild TBI contributed to this improvement RPQ scores. Based on the findings described here, HBOT confers great benefits by improving the quality of life of patients with mild TBI and likely prevents further brain damage by increasing cerebral blood flow, improving neuropsychological disorders, and promoting neurophysiological and electrophysiological recovery. Given that repeated HBOT sessions restored neurological deficits and cognitive impairment, HBOT should be incorporated as a therapeutic modality for the treatment of patients with head injuries. Although there are many benefits of HBOT, there are also several drawbacks such as seizures, oxygen poisoning, pneumothorax, and middle ear injuries.
Although this study supports the efficacy of HBOT in treating post-concussion syndrome, the small sample population and single biomarker are limitations that must be addressed in future studies. The kinetics and underlying mechanisms of HBOT also warrant further investigation to maximize the many beneficial effects of HBOT.
HBOT effectively relieves the symptoms associated with post-concussion syndrome through a mechanism that involves repressing MMP-9 activity.
Written informed consent for publication of the patients’ details was obtained from the patients/a guardian of the patient.
Zenodo: Hyperbaric oxygen therapy ameliorates the symptoms of post-concussion syndrome by inhibiting MMP-9 activity: A Randomized Controlled Trial in Indonesia. http://doi.org/10.5281/zenodo.4785703.46
This project contains the following underlying data.
This project also contains the following reporting guidelines.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hyperbaric oxygen therapy, brain, traumatic brain injuries
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: TBI, HBOT, mild TBI, Rehabilitation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Jun 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
1. The predominant age group in this study (around 60), also I ... Continue reading It' s an interesting study to see the impact of HBOT. Several concern regarding the result are:
1. The predominant age group in this study (around 60), also I would like to see the mechanism of injury.
2. Laboratory result often not associated with clinical features of patients. I would like to see the clinical characteristic of the patient before and after HBOT, such as GCS, neurological function, etc. The clinical part will strengthen this study and should be addressed in the discussion.
3. The conclusion in this study probably better with minor revision. There are so many pathway that could be associated with this HBOT treatment for concussion, and one of them is associated with MMP family. We should not certainly say "thorough" one pathway in this study.
Overall, I still consider this is an interesting study for traumatic brain injury.
1. The predominant age group in this study (around 60), also I would like to see the mechanism of injury.
2. Laboratory result often not associated with clinical features of patients. I would like to see the clinical characteristic of the patient before and after HBOT, such as GCS, neurological function, etc. The clinical part will strengthen this study and should be addressed in the discussion.
3. The conclusion in this study probably better with minor revision. There are so many pathway that could be associated with this HBOT treatment for concussion, and one of them is associated with MMP family. We should not certainly say "thorough" one pathway in this study.
Overall, I still consider this is an interesting study for traumatic brain injury.