Keywords
anaphase-promoting complex, mitotic cyclin, complex haploinsufficiency, cell division, dominant negative mutant protein
anaphase-promoting complex, mitotic cyclin, complex haploinsufficiency, cell division, dominant negative mutant protein
Haploinsufficiency is a frequently observed phenomenon defined by the inability of a heterozygous mutant locus to produce the same phenotype as the corresponding homozygous wild-type locus in a diploid organism. Since it was first proposed in 1932 by O. L. Mohr (Stern 1943), the word “haploinsufficiency” has long been used to simply describe the dosage effect of one or more heterozygous loci. However, in some cases, two heterozygous loci co-existing in the same organism produce a phenotype not seen in either of the corresponding single heterozygotes, a phenomenon defined by Haarer et al. (2007) as complex haploinsufficiency. Establishment of a complex haploinsufficient relationship among genes can help reveal the structure of a genetic network in a biological process especially when lethality prevents the generation and/or analysis of a double mutant of two loci.
Cell cycle regulators form tightly knit networks in eukaryotes (Li et al. 2004; Giotti et al. 2017; Van Leene et al. 2010). However, depending on the circumstances, functional studies of the network of cell cycle regulators in development of complex multicellular organisms are hindered by either lethality of single mutants (Murphy et al. 1997; Liu and Finley 2010; Wang et al. 2012; Wang et al. 2013; Guo et al. 2016) or non-existence of a mutant phenotype likely due to redundancy of homologous genes (Jacobs et al. 1998; Ye et al. 2001; Pérez-Pérez et al. 2008). In particular, a mutant phenotype for a mitotic cyclin in embryo development and seed germination has not been observed in plants.
The genome of Arabidopsis contains 87 core cell cycle regulators, including 10 A-type and 11 B-type mitotic cyclins (Menges et al. 2005). No mutants of these cyclins have been shown to produce an embryo-defective or a seed-germination-defective phenotype, even though gene expression studies have implicated a role in embryo development and/or seed germination for most of them, including TARDY ASYNCHRONOUS MEIOSIS (TAM)/CYCA1;2 (Masubelele et al. 2005; Narsai et al. 2011; Hofmann et al. 2019). Homozygous tam-1/tam-1 and tam-2/tam-2 mutants are defective in meiotic cell cycle progression and in nuclear size regulation in trichomes and guard cells, but no obvious defect was observed in their embryo development and seed germination (Magnard et al. 2001; Wang et al. 2004; Wang et al. 2010; Jha et al. 2014; Wang and Yang 2014). The previous studies also did not reveal any defect in heterozygous TAM/tam-1 and TAM/tam-2 plants. Thus, it is likely that functional redundancy occurs among TAM and other mitotic cyclins in embryo development and seed germination.
Another major cell cycle regulator, the anaphase-promoting complex/cyclosome (APC/C), is a large E3 ubiquitin ligase complex that mediates the ubiquitination of mitotic cyclins and other cell cycle regulators, which leads to degradation of these regulators by the proteasome (Sudakin et al. 1995; Irniger et al. 1995; King et al. 1995; Yamano 2019). Degradation of cell cycle regulators via the APC/C in a temporal order allows the cell cycle to exit mitosis and prevents the reentry into the next S-phase of the cell cycle (Yamano 2019). On the other hand, cyclin As and/or Bs activate the APC/C via phosphorylation of the APC3 and APC1 subunits or an inhibitor of the APC/C activator CDC20 by cyclin-CDK complexes (Lahav-Baratz et al. 1995; Kramer et al. 2000; Rudner and Murray 2000; Dienemann and Sprenger 2004; Zhang et al. 2016). Cyclin As can also inhibit premature activation of the APC/C by phosphorylating the APC/C activators CDH1 and CDC20 (Sørensen et al. 2001; Hein and Nilsson 2016). Although TAM has not been demonstrated to be a substrate of the APC/C, the CDKA1-TAM complex can phosphorylate in vitro OSD1, a negative regulator of the APC/C in Arabidopsis (Iwata et al. 2011; Cromer et al. 2012). However, in Arabidopsis, unlike single mutants of mitotic cyclins, several single mutants of subunits of APC/C are gametophytic-lethal or embryo-lethal (Capron et al. 2003; Kwee and Sundaresan 2003; Wang et al. 2012; Wang et al. 2013; Guo et al. 2016). In particular, embryo development is arrested at the zygote stage in the homozygous apc11-1/apc11-1 and apc11-2/apc11-2 mutants of the APC11 subunit of the APC/C although heterozygous APC11/apc11-1 and APC11/apc11-2 embryos develop normally (Guo et al. 2016). The zygotic lethality of the apc11 mutants hinders the study of the effect of the mutations on subsequent development in Arabidopsis.
In an attempt to investigate how TAM and APC11 genetically interact in Arabidopsis development, we conducted genetic crosses between two tam mutants and two apc11 mutants and between a transgenic line containing the TAM:TAM-GFP transgene and the apc11 mutants. Our findings revealed a strong complex haploinsufficient relationship between TAM and APC11 in both embryo development and seed germination, and a dominant-negative effect arising from the coexistence of the apc11-1 and the tam-1 alleles or of the apc11-1 allele and the TAM:TAM-GFP transgene.
The origins and initial characterization of the mutants and the TAM:TAM-GFP line used in this investigation were as described in our previous work (Magnard et al. 2001; Wang et al. 2004; Wang et al. 2010; Guo et al. 2016). Briefly, tam-1 and apc11-1 harbor point mutations that were generated by chemical mutagenesis (Magnard et al. 2001; Guo et al. 2016), and tam-2 and apc11-2 harbor T-DNA insertions (Wang et al. 2004; Guo et al. 2016), in the TAM and APC11 loci, respectively. The TAM:TAM-GFP line has been characterized with respect to its expression in male meiocytes and vegetative organs and its complementation of the meiotic defect in tam-1 (Wang et al. 2004; Jha et al. 2014). All seeds were air dried at least two weeks before sowing. The plants were grown in soil (Sunshine MVP growing Mix, Sungro Horticulture, Agawam, Massachusetts, USA) or on a medium containing 4.3 g/L Murashige and Skoog (MS) salt base (catalogue number 11117-074, Gibco, Waltham, MA, USA), 1% (w/v) sucrose (catalogue number S1695, Spectrum Quality Products, Gardena, CA, USA), and 0.7% (w/v) agar (catalogue number PTC001, Gaisson Laboratories, Smithfield, UT, USA) in a growth chamber at ~22°C. Daily light regime in the growth chamber was 16h fluorescent light supplemented with tungsten light (light intensity = ~50 µmol·m-2·s-1 near the plants) and 8h darkness.
Six types of crosses between a heterozygous apc11 mutant and a homozygous tam mutant or the TAM:TAM-GFP line were conducted. Buds of the tam mutants and the TAM:TAM-GFP line that were about to open were emasculated and their stigmas pollinated with pollen from the APC11/apc11-1 or APC11/apc11-2 plants. At least three fertile siliques were obtained from each type of the crosses. Care was taken to remove other buds from the same inflorescences to facilitate harvesting the seeds from the crosses.
The imbibed seeds were dissected with a pair of 30G½ syringe needles under either a Leica S6D or a Nikon SMZ1000 dissecting microscope with a magnification range of 2x to 4x. Photographs of the seeds were taken using the Leica EC3 imaging system on the Leica S6D microscope. Embryos were observed and photographed on a Nikon Eclipse 80i microscope equipped with the differential interference contrast (DIC) optics and the Nikon DS-Ri1 imaging system. The images shown in the figures were lightly adjusted for contrast and brightness and made black and white on the entire image area in Adobe Photoshop CC 2014.
To investigate how apc11 mutants genetically interact with tam mutants, we crossed APC/11apc11-1 and APC11/apc11-2 individuals with tam-2/tam-2, respectively. Each of the crosses resulted in more than 50 F1 seeds. However, these seeds, after fully dried, failed to germinate in more than 14 days on the MS agar medium, in contrast with the corresponding wild-type (Col-0) seeds germinating within 2-3 days (Wang et al. 2016). The germination defect observed did not result from abnormally underdeveloped embryos because embryos dissected out from these seeds appeared fully developed (Figure 1). Because the pollen donor plants for these crosses, APC11/apc11-1 or APC11/apc11-2, were heterozygous for the apc11 mutation as the homozygous mutant plants are not available due to embryo lethality, the genotypes of the F1 seeds should be either APC11/APC11;TAM/tam-2, APC11/apc11-1;TAM/tam-2, or APC11/apc11-2;TAM/tam-2. Because seeds heterozygous for any of the three mutations alone did not exhibit such a severe germination defect (Wang et al. 2010; Guo et al. 2016), the germination defect observed indicates complex haploinsufficiency between apc11-1 and tam-2, and apc11-2 and tam-2. Moreover, because all the F1 seeds failed to germinate and approximately half of their embryos should have the genotype of APC11/APC11;TAM/tam-2, our results suggest that APC11 was also insufficient even in the APC11/APC11;TAM/tam-2 embryos.
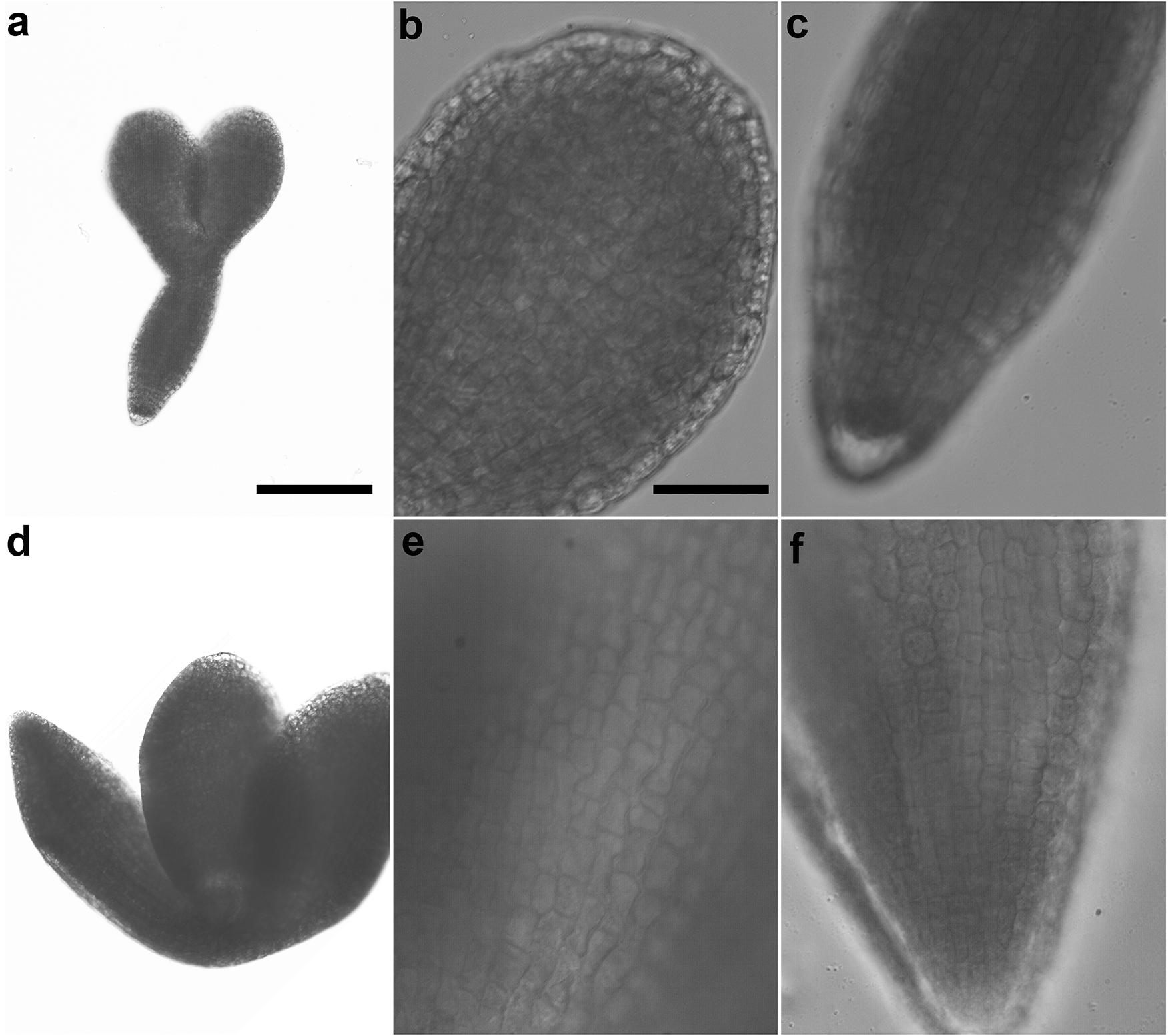
(a-c) From a cross between APC11/apc11-1 and tam-2/tam-2. (a) A representative embryo from an imbibed seed that failed to germinate. (b) and (c) Close-up views of a cotyledon and the root of the embryo in (a), respectively. (d-f) From a cross between APC11/apc11-2 and tam-2/tam-2. (d) A representative embryo from an F1 seed. (e) and (f) Close-up views of a cotyledon and the root of the embryo in (d), respectively. The embryo in (d) was larger than that in (a) presumably due to an unreduced egg frequently resulting from the tam-2 mutation (Wang et al. 2010; d'Erfurth et al. 2010). Scale bar in (a) for (a) and (d) = 200 µm, and scale bar in (b) for (b), (c), (e), and (f) = 50 µm.
To provide additional evidence for the complex haploinsufficient relationship between APC11 and TAM, we crossed the APC11/apc11-1 and APC11/apc11-2 plants with another mutant, tam-1/tam-1, respectively. The tam-1 mutation is a point mutation that does not result in unreduced gametes as tam-2 and it produces normal diploid embryos and germinated seeds (Wang et al. 2004; Wang et al. 2010). Surprisingly, F1 seeds from the crosses between APC11/apc11-1 and tam-1/tam-1 varied in size and showed variable degrees of shrinkage (Figure 2a). These seeds also failed to germinate like the F1 seeds from the crosses between APC11/apc11-1 and tam-2/tam-2. However, unlike the F1 seeds from the crosses between APC11/apc11-1 and tam-2/tam-2, small and abnormal embryos were readily found after dissection of these seeds (Figure 2c-f) even though some embryos were morphologically normal (Figure 2f). These observations indicate that apc11-1 and tam-1 have a complex haploinsufficient relationship in embryo development and seed germination, given that APC11/apc11-1 and TAM/tam-1 individuals do not have such defects (Magnard et al. 2001; Wang et al. 2004; Guo et al. 2016). The level of complex haploinsufficiency between apc11-1 and tam-1 is more severe than those between the two apc11 mutants and tam-2. The apc11-1 and apc11-2 mutations are also a point mutation and a T-DNA insertion mutation, respectively, with apc11-2 having a more severe embryo defective phenotype than apc11-1 (Guo et al. 2016). Therefore, the most severe complex haploinsufficiency was found with two relatively weak point-mutation alleles.
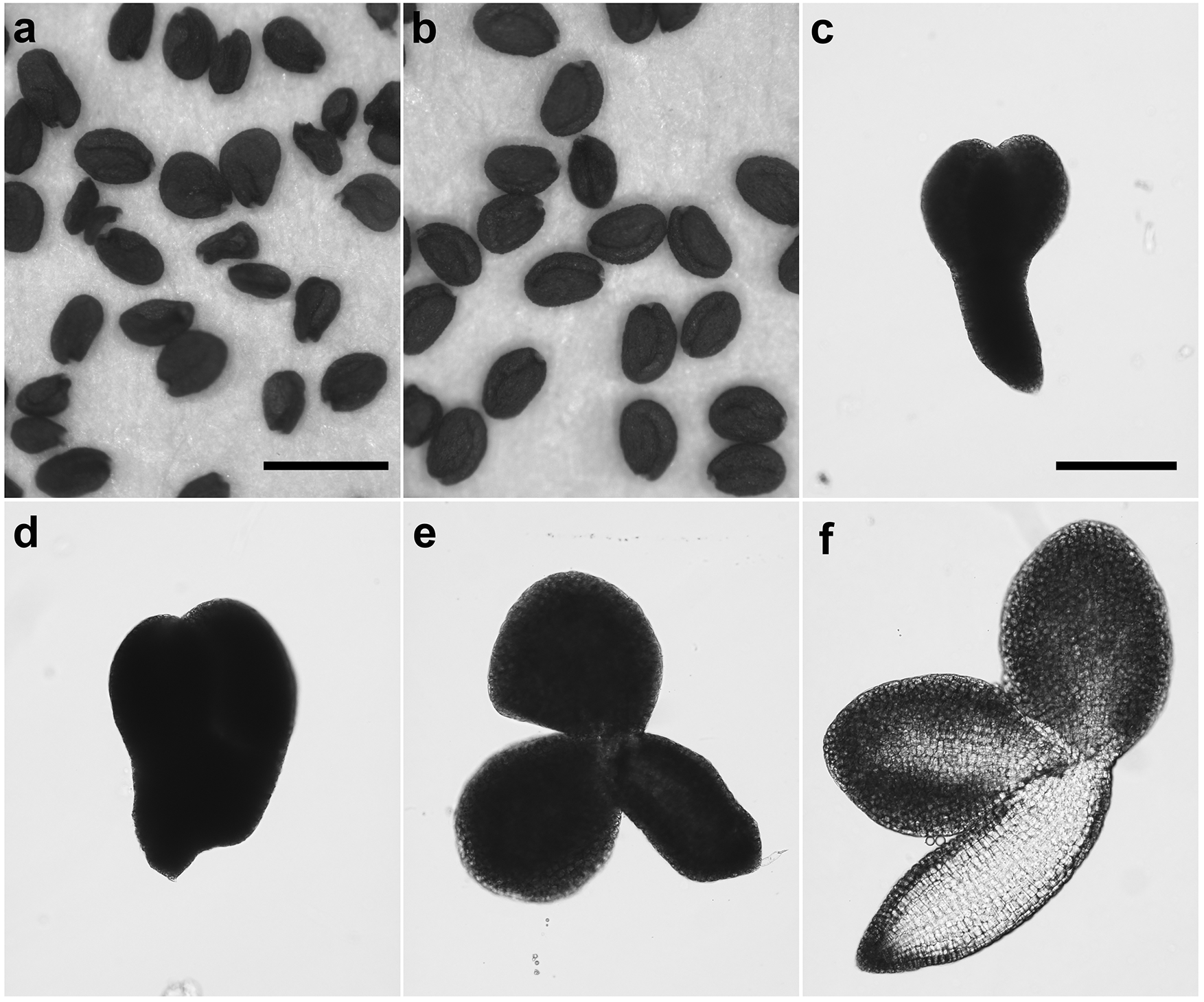
(a) and (c-f) From a cross between APC11/apc11-1 and tam-1/tam-1. (a) Seeds of variable sizes and morphologies. (c-f) Embryos from imbibed seeds that failed to germinate, showing variable sizes and morphologies. The variation in seed or embryo size should not have resulted from variable ploidy levels of the egg cells because the tam-1 mutant, unlike the tam-2 mutant, produces only haploid gametes (Magnard et al. 2001; Ajay et al. 2014). (b) Seeds from a cross between apc11-2 and tam-1. Scale bar in (a) for (a) and (b) = 1 mm, and scale bar in (c) for (c-f) = 200 µm.
The F1 seeds from the cross between APC/11/apc11-2 and tam-1/tam-1 appeared normal (Figure 2b) and germinated and developed into mature plants. This result is consistent with tam-1 being a mild allele that did not result in complex haploinsufficiency in the double heterozygous F1 individuals during embryo development and seed germination.
In an attempt to investigate how the TAM protein is affected in the apc11 mutants, we crossed APC11/apc11-1 and APC11/apc11-2 with a homozygous TAM:TAM-GFP line in the Col-0 wild-type background, TAM-GFP/TAM-GFP, respectively. Surprisingly, for each type of the crosses, none of the more than 30 F1 seeds germinated after two weeks on the MS agar medium. Seeds from the crosses between APC11/apc11-1 and TAM-GFP/TAM-GFP appeared small andshrank (Figure 3a). Dissection of their non-germinated seeds revealed that the embryos were arrested at either theapical cell stage (Figure 3b) or the early globular stage (Figure 3c). Most of the F1 seeds from the crosses between APC11/apc11-2 and TAM-GFP/TAM-GFP were similar in size to normal seeds in two dimensions (length and width) but abnormally thin in the third dimension (depth), i.e., with a flattened appearance (Figure 3d). Some of these seeds were apparently abnormally small (Figure 3d). Dissection of the non-germinated seeds produced embryos at an advanced developmental stage (Figure 3e-g). The TAM:TAM-GFP transgene alone does not cause an embryo developmental arrest or a seed non-germination defect (Wang et al. 2004). Therefore, the defects observed suggest a dominant negative effect of the TAM-GFP protein in the F1 seeds where the APC11 protein was presumably reduced. In fact, these defects parallel those of the corresponding F1s from the crosses between APC11/apc11-1 and tam-1/tam-1, and between APC11/apc11-2 and tam-2/tam-2, respectively, although they were more severe with the TAM:TAM-GFP transgene than with the tam mutations.
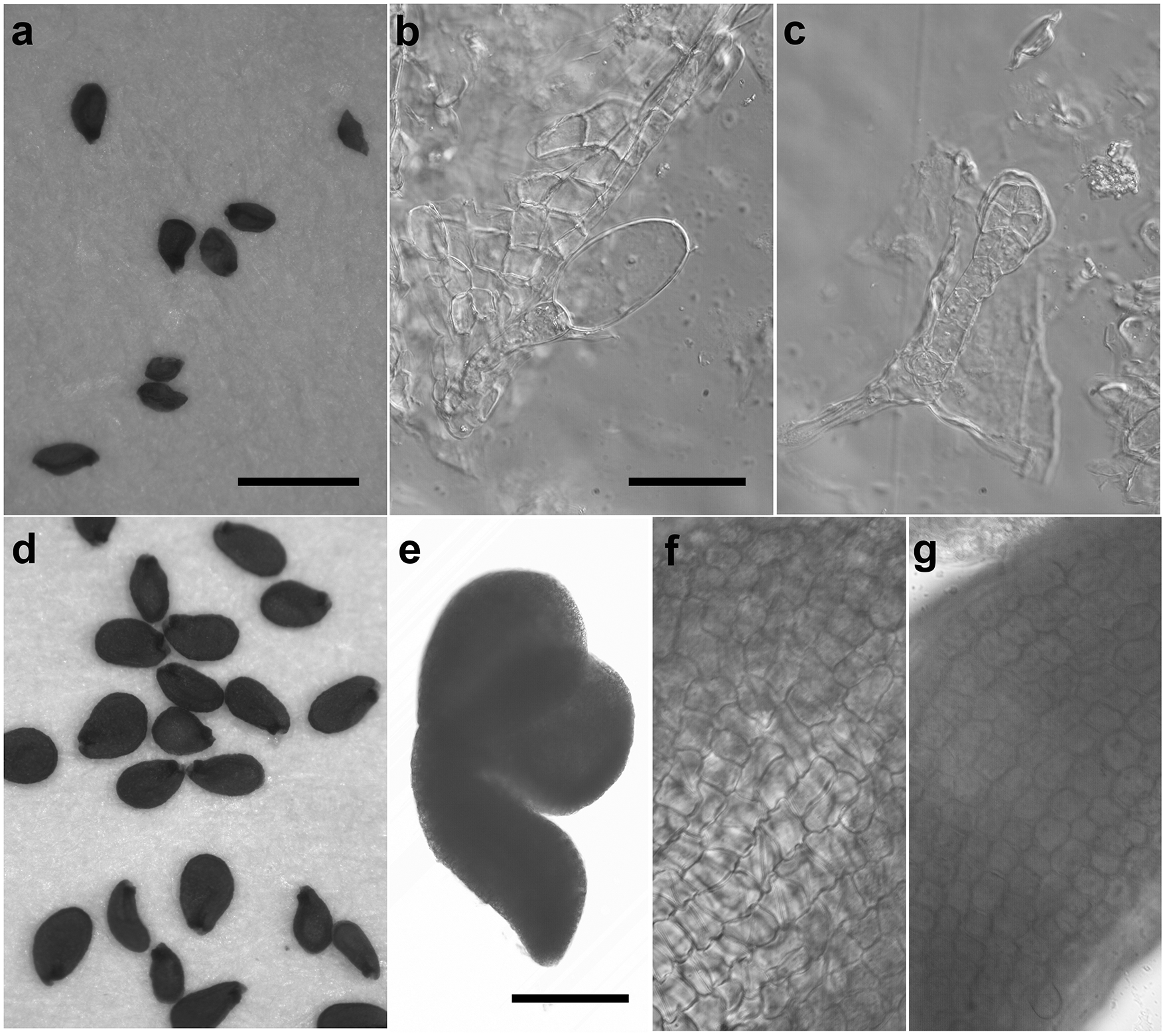
(a-c) From a cross between APC11/apc11-1 and TAM-GFP/TAM-GFP. (a) Small and shriveled seeds. (b) A developmentally arrested embryo from an imbibed seed that failed to germinate, showing an enlarged apical cell attached to the suspensor. (c) A developmentally arrested embryo from an imbibed seed that failed to germinate, showing an early globular embryo attached to the suspensor. (d-g) From a cross between APC11/apc11-2 and TAM-GFP/TAM-GFP. (d) Flattened seeds. (e) A representative embryo from an imbibed seed that failed to germinate. (f) and (g) Close-up views of a cotyledon and the root of the embryo in (e), respectively. Scale bar in (a) for (a) and (d) = 1 mm, scale bar in (e) = 200 µm, and scale bar in (b) for (b), (c), (f) and (g) = 50 µm.
Our findings of complex haploinsufficiency between pairs of apc11 mutations and tam mutations in both embryo development and seed germination are consistent with the knowledge that APC/C and mitotic cyclins reciprocally regulate each other’s functions in cell cycle progression in eukaryotes. The role of TAM in embryo development and seed germination and the role of APC11 in seed germination have not been reported before. Therefore, testing if two mutations have a complex haploinsufficient relationship is a useful approach for functional studies of genes acting in embryo development and/or seed germination. This approach can work for a mutant such as tam-1 or -2 that by itself does not have an obvious defect in embryo development and seed germination. It can also work for an embryo lethal mutant such as apc11-1 or -2 for demonstrating its effect on seed germination.
In Arabidopsis, there are many known recessive embryo lethal mutants (Meinke 2020) and other recessive mutants (e.g. mutants available from the Arabidopsis Biological Resource Center, Columbus, OH) that do not exhibit defects in embryo development and/or seed germination and yet their wild-type genes are expressed in the embryo (Narsai et al. 2011; Hoffmann et al. 2019). Any pairs of mutants from these two categories may be tested in the F1 generation for complex haploinsufficiency if the paired mutants are suspected to affect the same developmental process based on available information. This approach should circumvent the difficulty of identifying the double mutant of such a pair of mutants and be faster than the double mutant analysis because it involves only the F1 seeds.
The tam-2 and apc11-2 alleles are more severe alleles than the tam-1 and apc11-1 alleles, respectively (Magnard et al.; zWang et al. 2004; Wang et al. 2010; Guo et al. 2016), but the defects exhibited in the F1 seeds of this investigation were most severe between tam-1 and apc11-1, comparing to between tam-2 and apc11-2, tam-1 and apc11-2, and tam-2 and apc11-1. The tam-2 and apc11-2 alleles are T-DNA insertion mutants that likely do not produce the TAM and APC11 proteins, whereas the tam-1 and apc11-1 alleles are expected to produce the mutant proteins because of the nature of their point mutations generated from chemical mutagenesis. Therefore, the presence of both mutant proteins correlates with the most severe defects in comparison with the other combinations without the presence of both mutant proteins. This phenomenon, in principle, may be explained by reciprocal dominant-negative effects of the apc11-1 and the tam-1 mutant proteins. As discussed earlier, both positive and negative interplays exist between the APC/C and mitotic cyclins. This relationship may underpin the phenomenon that the presence of both mutant proteins has a greater functional impact than the presence of one, or absence of both, of the mutant proteins. Interestingly, the TAM:TAM-GFP line produced even more severe complex haploinsufficient-like phenotypes than tam-1 and tam-2. This finding further supports the idea that the presence of a malfunctioned protein, which is assumed to be the case for the TAM-GFP protein, is more devastating to the plant than without such a protein, especially when in coexistence with the apc11-1 mutant protein.
Because the crosses in the current investigation were conducted with APC11/apc11-1 and APC11/apc11-2, approximately one half of the F1 plants should be double heterozygous for the apc11 and tam mutations and the other half homozygous wild type at the APC11 locus and heterozygous for one of the tam mutations. Similar allele frequencies should occur in F1s of the crosses between the apc11 mutants and the TAM:TAM-GFP line. However, when a defect was present, it was observed in all F1 seeds of the crosses albeit the severity of embryo developmental defects, if existed, varied. This observation indicates that reconstituting homozygous APC11 from fertilization could not rescue the F1s from abnormal embryo development or seed non-germination in the TAM/tam background. A plausible explanation for this outcome in the F1s is that the pollen grains used in the crosses had a reduced level of the APC11 protein or APC11 transcript due to heterozygosity at the APC11 locus in the parental tissue, and the APC11 protein or APC11 transcript could not recover to the normal level in the haploid pollen grains even though they had a wild-type allele of APC11. If so, embryo development and seed germination seem to be sensitive to simultaneous reductions in APC11 and TAM levels or to a reduction in the APC11 level along with the presence of a malfunctioned TAM-GFP.
The roles of genes encoding core cell cycle regulators in Arabidopsis seed germination have been demonstrated for two D-type cyclins genes, CYCD4:1 and CYCD1;1 (Masubelele et al. 2005). Expression of these genes was found to be activated before the onset of cell division in the radicle, and the earliest cell divisions in the radicle preceded root emergence from the seed coat that defines the completion of germination. Cell divisions in the radicles of the cycd4:1 and cycd1;1 mutants were reduced, which correlated with the delayed root emergence from their seed coats (Masubelele et al. 2005). Interestingly, activation of expression of two A-type cyclin genes, one of which is TAM (the other CYCA3:4), was also found along with the earliest expressed D-type cyclin genes (Masubelele et al. 2005). The findings from this investigation support the roles of these A-type cyclins in seed germination. Furthermore, the non-germination phenotype associated the complex haploinsufficiency lends support to the view that cell divisions are required for seed germination. Detailed genetic analysis with combinations of the D-type and A-type cyclin mutants may shed more light on the essential role of cell division in seed germination in Arabidopsis.
Figshare: Complex haploinsufficiency between TAM and APC11.
https://doi.org/10.6084/m9.figshare.13574891.
This project contains the following underlying data:
Image files of F1 dry seeds and imbibed non-germinated seeds, and embryos dissected from imbibed non-germinated seeds.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
MY conceived the idea and designed the experiments. MY and YW performed the experiments. MY analyzed the experiments. LG and C-ML contributed the apc11 mutants. MY wrote the manuscript with input from YW, LG, and C-ML.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant molecular genetics, plant hormones, plant stress responses.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Genetics and seed molecular biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Jan 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)