Keywords
Field Implementation, environmental enteropathy, biomarkers, data collection, malnutrition, stunting, microbiome
Field Implementation, environmental enteropathy, biomarkers, data collection, malnutrition, stunting, microbiome
There are no major edits in the current version. Some minor edits have been done based on reviewer feedback, including changes in Figures 2 and 3.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
AKU: Aga Khan University
CHWs: Community Health Workers
EE: Environmental Enteropathy
ENA: Emergency Nutrition Assessment
ERC: Ethics Review Committee
HAZ: Height-for-Age
HFIAS: Household Food In-security and Access Scale
IDRL: Infectious Diseases Pediatric Research Laboratory
IMCI: Integrated Management of Childhood Illnesses
INR: International Normalized Ratio
L:R: Lactulose:Rhamnose
LMICs: Low- and Middle-income countries
MAL-ED: Malnutrition and Enteric Disease Study
MAM: Moderate Acute Malnutrition
MUAC: Mid Upper Arm Circumference
RUSF: Ready-to-use Supplementary Food
RUTF: Ready-to-use Therapeutic Food
SAM: Severe Acute Malnutrition
SEEM: Study of Environmental Enteropathy (EE) and Malnutrition
UGI: Upper Gastrointestinal
UNICEF: United Nations International Children's Emergency Fund
WAZ: Weight for Age
WHO: World Health Organization
WHZ: Weight-for-Height
Malnutrition is defined as an imbalance of nutrient intake that results in either overnutrition (manifested as obesity) or undernutrition (manifested as stunting, wasting, and/or underweight). Undernutrition is directly or indirectly responsible for almost half of deaths in children under five years of age in low and middle-income countries (LMIC) with severe implications for child survival and individual intellectual development, and adverse national economic consequences. Southeast Asia carries half of the global burden for stunting (length for age Z score<-2; 22%) and wasting (weight for length Z score<-2; 7.5%) in children under five years of age.1 Stunted Pakistani children comprise ~45% of all stunted South East Asian children, attributing to the third-highest percentage of stunting worldwide.2 Among various factors, poverty and food insecurity in these areas are the main risk factors for undernutrition, which is further aggravated by poor sanitation and unhygienic living conditions. Stunting is mediated in large part by EE, a subclinical disorder characterized by non-specific intestinal inflammation with or without overt diarrhea episodes.3 It is a process driven by repeated exposure to entero-pathogens, and exposure to environmental contaminants/toxins. The hallmarks of EE pathophysiology are believed to be gut barrier dysfunction, non-specific inflammation, and malabsorption.
We implemented SEEM in the rural district of Pakistan; the primary objective was to test the biomarkers of EE in malnourished and well-nourished children. Matiari is a rural district that is 185km/115 miles north of the city Karachi, where the Aga Khan University (AKU) flagship campus is based. Its total population is 0.769 million with approximately 143,000 households in around 1600 villages.4 The major source of economic growth in this area is from agriculture-related activities. Matiari is subdivided into 18 Union Councils (the equivalent of municipalities in the United States), which are its administrative subunits. The average population per union council is 30,000 people with an average household size of 6.7 persons. The Department of Pediatrics and Child Health of AKU has been conducting community-based research projects in this district since 2002 and our research team has successfully implemented several large community-based trials on maternal, neonatal, and child health.5–15 In our current study, SEEM, collection of field data and laboratory samples (blood, urine, and stool) involved traveling to the residence of the families versus the use of a field office as a central sample and data collection point. This decision was driven by feasibility given that our study population primarily lived in widely scattered villages from where access was limited due to poor road and public transportation infrastructure. It was logistically difficult to ask parents to bring their children to a field office or any fixed facility for sample collection.
The Matiari district shoulders an immense burden of maternal, neonatal, and childhood illnesses as evidenced by a stillbirth rate of 42·8 (40·5–45·2)/1000 total births, neonatal mortality of 46.9 (44.4–49.4)/1000 live births, and a maternal mortality ratio of 259 (198–319)/100,000 live births.16 The Matiari site has a field laboratory with basic equipment needed for processing biological urine, blood, and stool samples and is staffed by technicians trained at AKU that receive periodical refresher training via regular visits to our central research coordinating facility - the AKU Infectious Diseases Pediatric Research Laboratory (IDRL) at Karachi. The site is also equipped with an electronic Data Management Unit (DMU) which enables both electronic transcriptions of the data collected in the field on hard/paper copies, and direct electronic data collection via portable tablets through customized applications. Furthermore, the DMU has data storage servers that enable data synchronization with central servers at AKU, Karachi. We have previously successfully conducted several studies on maternal and child health at this study site.5–15 However, we found SEEM to be unique in terms of the additional biological specimens and data collection processes we had to implement given that these samples would then be processed for high throughput sensitive ‘omics analyses such as microbial RNA and metabolomics.
The study protocol was approved by the Aga Khan University Ethics Review Committee (ERC, 3836-Ped-ERC-15) in December 2015, and the study is registered on clinicaltrials.gov (ID: NCT03588013).17
The primary objective of the SEEM study was to understand the clinical phenotypes of children at risk for EE in order to identify predictive ‘omic profiles along with detailed microbiome and transcriptomics as potential therapeutic targets. A cohort of 416 children was selected for a longitudinal study; 365 of whom were diagnosed with acute malnutrition or wasting (defined as weight-for-height Z (WHZ) score < -2) while 51 healthy controls or well-nourished children (defined as WHZ score ≥ 0 and height-for-age Z (HAZ) score ≥ -1 on two consecutive follow-ups from three to six months) were recruited. Our detailed study methods and results have been published.18,19 In brief, birth surveillance was established in which parents registered their newborns within 30 days of birth followed by monthly anthropometric assessment until six months of age. Between the time of birth registration and six months of age, data regarding young infant feeding practices, morbidity, and health-seeking behavior in the case of illness was also collected. Anthropometry measurements including height, weight, and mid-upper arm circumference (MUAC) of both parents and children, with an additional measure of head circumference were recorded from our sample of children. Anthropometry data collected between three and six months was used to calculate WHZ scores based on World Health Organization (WHO) growth reference standards;20 children were enrolled as cases and controls based on criteria defined above. At the time of enrollment, we collected data of the Household Food Insecurity and Access Scale,21 illnesses and treatment received, socioeconomic status, maternal and paternal education (Table 1). Enrolled children were then followed weekly for morbidity data (diarrhea, respiratory tract infection, antibiotic use, and hospitalization) and monthly for anthropometric measurements. Blood, stool, and urine samples were collected at the time of enrollment and at nine months of age for biomarker assessment. Breast milk samples from mothers were also collected once at the time of enrollment for oligosaccharides assessment.
Between six and nine months of age, nutritional education counseling was provided monthly to cases i.e. acute malnourished children. At nine months, if the child had a persistent WHZ score < -2, a chick-pea-based and locally made Ready-to-use Supplementary Food known as AchaMum was initiated for two months as per WHO guidelines for the management of moderate acute malnutrition.22 For severe acute malnutrition, however, while the WHO recommendation is for Ready-to-use Therapeutic Food (RUTF), we provided AchaMum according to a child’s weight (similar to RUTF) due to logistic and administrative issues in importing RUTF. If the child’s WHZ score consistently remained < -2 after the intervention, he/she was referred for further clinical assessment by a pediatric gastroenterologist for the screening of celiac disease and detailed luminal evaluation via an upper gastrointestinal (UGI) endoscopy.
Implementation challenges:
1. Collection of 24 hours food intake frequency data
A 24 hours food recall data was collected through a specialized structured instrument designed to collect all food items consumed by the child in the last 24 hours. This provided a quantitative estimate of the non-breast milk food intake. The form was administered between six and 24 months on every alternate month. This data was collected by an experienced dietitian or an individual with knowledge regarding food preparation techniques, portion sizes, recipes, and food intake. Six community health workers (CHWs) conducted the dietary intake form after getting training in the Naushahro Feroze district, an area where a similar food recall form was administered as part of the Malnutrition and Enteric Disease Study (MAL-ED) cohort.23
As the mothers in this area participate in fieldwork including crop harvesting, it was challenging to find optimal times to contact and interview them. We also found that some families temporarily migrated to different villages in response to employment opportunities. If a new recipe was found, which was not mentioned in the existing MAL-ED coding list, then CHWs cooked these recipes in the field office kitchen with the same method as cooked per the report by the mothers. The purpose of this activity was to identify the caloric value of each food item mentioned in the recipe. It was not only the data collection that was challenging but converting this data into a quantitative form for data analysis was a huge task. For this, the CHWs had to work for several hours on every single form to search for the codes already assigned to food items by the MAL-ED team and to assign new codes for new food items.
2. Management of acute childhood illnesses
All sick enrolled children were visited at their homes and assessed by study physicians as per Integrated Management of Childhood Illnesses guidelines.24 Medications were prescribed for mild-to-moderate and serious illnesses, the child was referred to the nearest healthcare facility with facilitated transport.
3. Anthropometry measurements and quality assurance
Weighing machines were monitored by daily standardization through calibrated weights of 0.5,1, 2, and 5 kilograms. Performance of CHWs was monitored in several ways: 1) standardization sessions were performed every three months to ensure reliability, precision & accuracy; 2) repeated measurements were taken from each child to increase the accuracy and precision, for 3-5% of the participants these repeat measurements were performed within 24-72 hours of the first measurement; 3) if measurements differed between two consecutive follow-ups, these were anthropometric measurements were recorded daily and outlier readings were then verified independently by the field team monitors, and 4) real-time feedback was provided to the field worker identified as responsible for the error. Furthermore, we employed the use of the Emergency Nutrition Assessment software (Action against Hunger, Canada)25 for digit preference of weight, height and MUAC measurements, and these scores were reviewed weekly.
4. Upper GI endoscopy consent
After a nutritional intervention, children who did not adequately respond (WHZ remained < -2) were thus selected for further evaluation. This included a detailed clinical workup and, if indicated, an upper GI endoscopy with biopsies as per pediatric gastroenterology guidelines.26,27 Challenges encountered in the group of children selected for UGI endoscopies included: 1) 13 out of 89 biopsies had to be postponed as children were deemed unfit for anesthesia (seven children suffered from flu, fever and runny nose; three suffered from low hemoglobin levels; one had diarrhea; one had low platelets, and in one case the mother was too ill to accompany her child to Karachi). Children with low hemoglobin were prescribed oral iron until their hemoglobin levels reached an acceptable level (i.e. hemoglobin >=9mg/dl) for procedure eligibility; 2) 13 children (12.7%) were determined to be ineligible due to pre-existing diseases and disorders, which included tuberculosis, neurological disorders, thalassemia, congenital anomaly and, epilepsy; and 3) obtaining consent for the procedure from the parents required need of an additional explanation of risks and benefits of the procedure. As per protocol, the parents were re-consented by the pediatric gastroenterologist when they arrived at the AKU Hospital before the procedure.
5. Laboratory samples collection challenges
Breast milk sample collection
Sugars found in breast milk are known to serve as prebiotics and help in establishing the health-promoting gut microbes in infants. The presence of female study physicians led to a reduction of anxiety and addressed the fears the mothers were harboring. The sample was transported to and stored at Matiari field laboratory at 2-8°C.
Stool samples collection for microbiome testing
Fecal sample collection for the purpose of microbiome analyses was performed for the first time in our study in a rural setting in Pakistan. This required the development of new feasibility measures and modification of transport protocols. An important aspect of fecal sample collection in the microbiome collection protocol, given the rapid degradation of bacterial RNA species at room temperature, includes a critical time window i.e. stool samples had to be collected and cryopreserved ideally within 30 minutes. This was particularly challenging given that the households could be up to 90 minutes’ drive from the field office. To allow rapid freezing of samples, dry cryo-shippers were procured to facilitate transportation back to the central IDRL at AKU, Karachi, a vehicle was modified to enable transportation of the field-site collection staff and the dry shipper along with the ability to park as close to the collection site as possible for appropriate rapid storage and cryo-preservation. The further challenges faced included multiple visits, at times due to diarrhea, insufficient quantity of stool produced and the challenge of sitting in open courtyards by CHWs and children during extreme weather conditions for several hours at a time. We found that the provision of access to a cellphone helped coordinate the best time for stool collection.
Urine sample collection for Lactulose: Rhamnose test
The lactulose: rhamnose (L:R) is a dual sugar absorption assay used to assess increased intestinal permeability as a surrogate measure of EE.28 The L:R test is performed by preparing oral lactulose and rhamnose solutions for the child to drink.29 Urine samples are obtained while fasting (before intake of lactulose and rhamnose), at 30, 60, and at 90 minutes after intake. These urine samples are then tested for the excretion of sugars.29 The biggest challenge was the collection of a fasting urine sample as it was difficult for mothers to keep their infants in a fasting state. The CHWs counseled and coordinated appropriate times for the collection of fasting samples and this aided in the sample collection. Additionally, the collection of urine samples at multiple time points required close monitoring. This process took several hours to complete. Furthermore, Matiari has average summer temperatures ranging from 38°C to 41°C,30 which leads to decreased urine sample collection in summer due to dehydration. This environmental limitation was a significant barrier in conducting the L: R sample collection.
We registered 2679 newborns within 30 days of their birth from March 2016 to October 2017. CHWs referred 712 children to study physicians between three and six months for inclusion in the study as malnourished children. The study physician enrolled 365 (51%) acute malnourished children in which laboratory specimens were collected in 346 (95%) children. Blood was collected in 341(93%) children, urine and stool/microbiome samples in 346 (95%) children, and breastmilk samples from mothers of 318 (87%) children (Figure 1). We also enrolled 51 well-nourished children in which we collected blood samples in 50 children, urine, stool and microbiome samples in 49(96%) children, and breastmilk samples from mothers of 48 (94%) children. We received a total of 6536 sick calls from the community from February 2017 to December 2018 and all sick children were visited by study physicians within 24-72 hours (Figure 2). From June 2016 to December 2018, we successfully filled 24 hours of food recall form in 90% of targeted follow-up visits (4612/5025). We collected 2656 stool and microbiome samples from enrolled children from the period of May 2016 to December 2018 and we were successfully able to preserve 73.2% of our samples in liquid nitrogen within 30 minutes of defecation (Figure 3). We attempted 311 urine L:R tests in which our success rate of collecting urine samples was 272(87%) with a 13% failure rate (Figure 4). We successfully carried out UGI endoscopies in 63 children out of 89 eligible children.
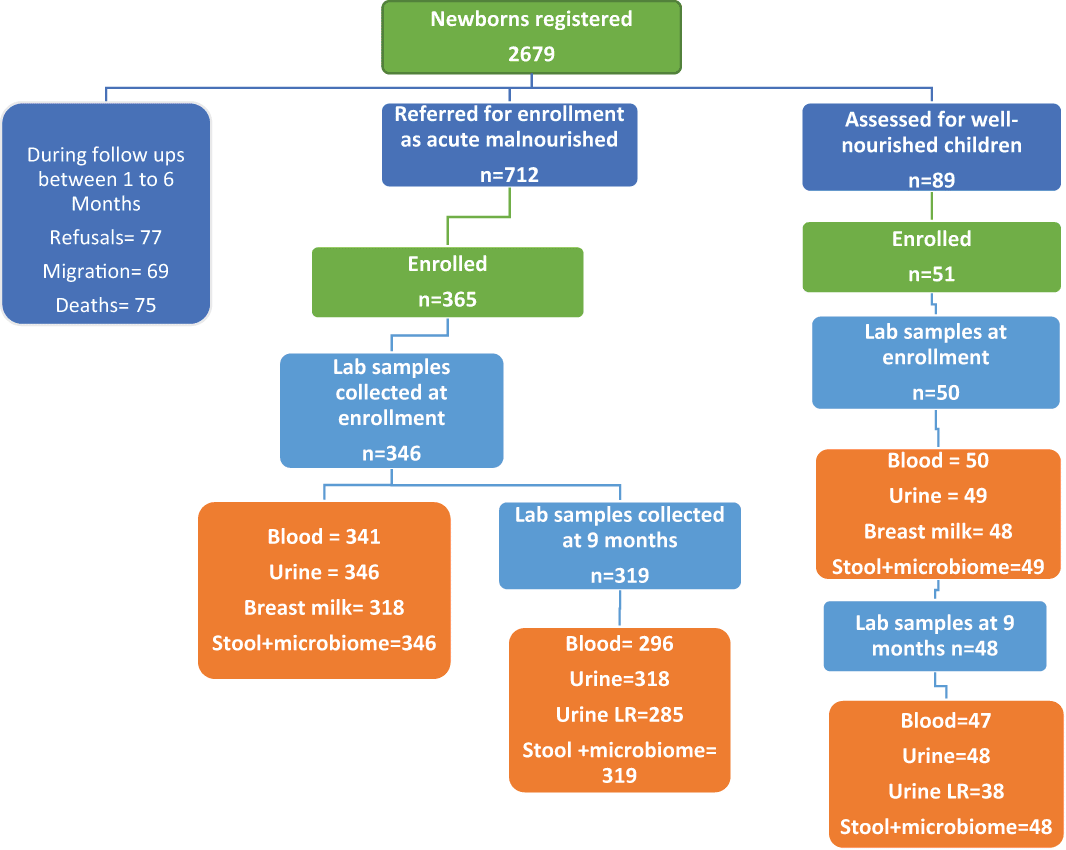
Participants flow diagram showing registration, enrollment and laboratory specimens’ collection at enrollment and then at 9 months of child’s health.

Month wise data of sick children visited by study physicians from February 2017 to December 2017 (total visits = 6536).
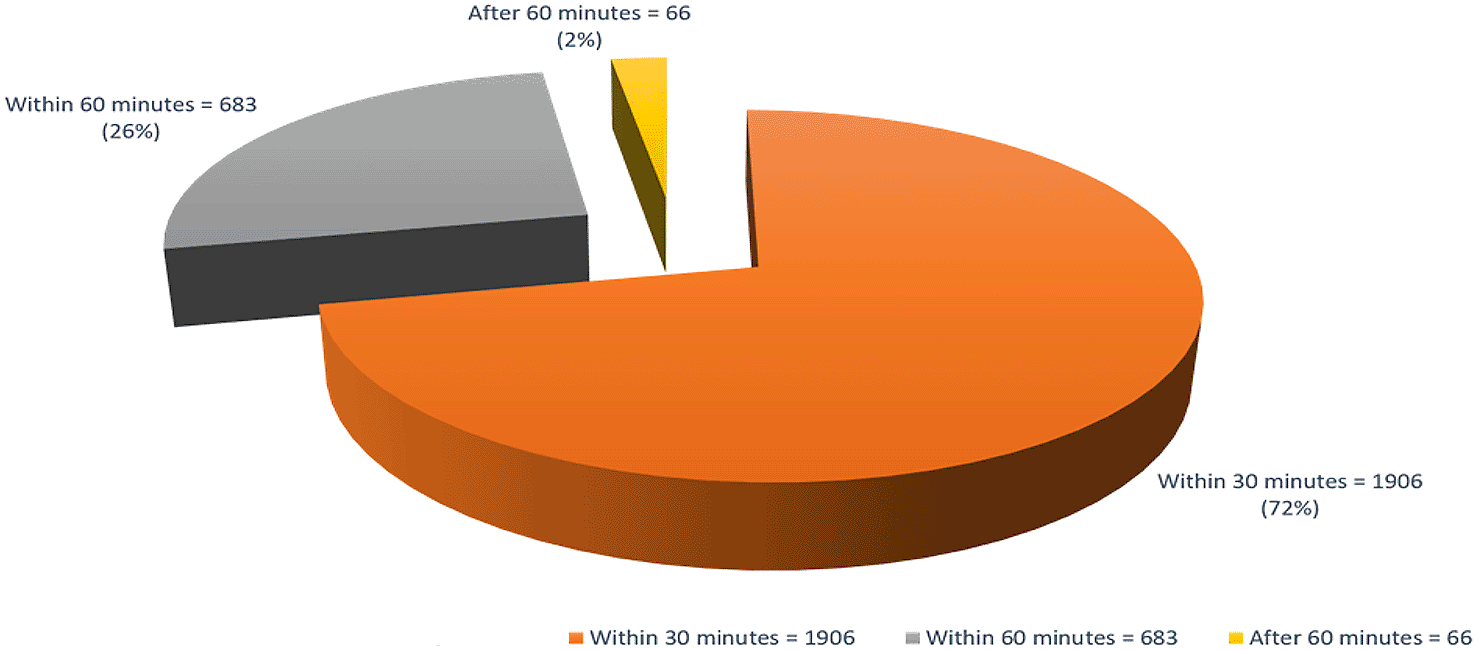
A pie chart showing the microbiome flash-frozen stool sample collection time difference in percentage (%) from the time stool passed (discovered by mother) to time placed in dry shipper from May 2016 to December 2018 (number of samples collected=2656).
The growing prevalence of undernutrition in LMICs has resulted in a high proportion of stunted and wasted children in these regions.1 EE, a subclinical disorder characterized by non-specific intestinal inflammation with or without overt diarrheal episodes, is a major contributor to stunting in this setting.3 SEEM has been implemented in rural Pakistan to advance our understanding of the pathophysiology of EE and to identify biomarkers related to the disease through following a birth cohort of malnourished and well-nourished children up to two years of age. Our group has faced several field challenges in implementing SEEM and this paper recognizes these challenges and highlights the field team’s efforts to successfully encourage participation and effectively and efficiently complete data collection. Further, it highlights the creation of an engaged study site in which the community was involved in the development of the study framework, in regard to data collection, to ensure efficient data and sample collection.
The field team was particularly successful in respecting parental time and comfort to collect data efficiently. For example, collecting data regarding food recall had proved challenging as the mothers were determined as the best respondents and their availability varied due to their occupation. Most of these women were involved in fieldwork, e.g. crop harvesting, and at times would have to move to a different region for farming-related reasons. Thus, scheduling interviews proved difficult, however, the field team created a data collection schedule determined on the mothers’ availabilities, therefore, ensuring effective food recall. Further, the field team maintained an understanding and respected that some mothers may be uncomfortable with sharing breastfeeding details or with how breast milk samples were handled, therefore female CHWs and physicians led this effort. Lastly, similar to previous studies,31 day-to-day variation in food recipes proved to be a limitation. We found that cooking food under supervision ensured the better collection of dietary recall data and breast milk samples and helped overcome this limitation.
Additionally, our field team also understood the social and economic restrictions that made it difficult for parents to bring their children to the AKU laboratory to provide samples. In lieu of this, the team visited the homes of participants to obtain the relevant data and samples; this team included an experienced phlebotomist to collect blood samples. Such efforts provided feasibility for the patients to participate in the study without adding a burden on the parents.
Another key challenge faced was the collection and transport of stool samples for microbiome analysis; appropriate sample acquirement and storage conditions affect the microbiome. Similar to Smith et al.,32 we devised a cryopreservation-based method to transfer the stool samples from Matiari to the AKU laboratory in Karachi. Our stool collection protocol consisted of cryopreserving the stool samples and transporting them in a specific vehicle suitable for dry shipment; this enabled long-term sample storage. Additionally, along with conducting necessary interviews and visits for data and sample collection, our team also counseled the parents regarding the microbiome and its importance – the parents were actively engaged, and the increased awareness made it easier to collect stool samples at the appropriate times.
Lastly, a unique aspect of our study was the use of electronic tablets for data collection. Most hospitals and research studies conducted in Pakistan make use of paper charts for all forms of data collection. Our use of electronic tablets was not unique for a rural setting but also enabled researchers to store data on a backend server which made data collection easier and that it would not be lost. Our experience and findings from this study can be replicated in similar research sites based in low- and middle-income countries not only in the context of environmental enteropathy but also in studies requiring community-based data and laboratory sample collection. This manuscript presents a summary of the challenges and lessons learned by our study staff in the implementation of field activities in accordance with the study protocol of SEEM and in the maintenance of continuous quality in-field data collection procedures. We found that efficient management and strong communication with the field staff allowed us to meet our goals of delivering quality samples leading us to achieve our study targets. Furthermore, regular training schedules on quarterly basis, a multilayered monitoring system, weekly progress meetings between the field staff, principal investigator and other collaborators led to the implementation of new field management protocols which allowed us to creatively solve the unique challenges and barriers encountered during this study. Our experience and findings from this study can be replicated to similar research sites based in LMICs not only in the context of EE but also in studies requiring community-based data and laboratory sample collection.
Aga Khan University Hospital ethical review committee approved the study in 2015 with ERC number 3836-Ped-ERC-15
Informed consent was received from parents/legal gaurdians of children for participation in this study and for their data to be published
All data underlying the results are available as part of the article and no additional source data are required.
The study was funded by the Bill and Melinda Gates foundation (AA: OPP1138727, SRM: OPP1144149. Dr. Asad Ali received the funding. The funding agency had no role in the design of the study and collection, analysis, and interpretation of data and in writing this manuscript.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to acknowledge mothers of enrolled children in Matiari for providing us time and data for this study. We would also like to thank all our CHWs for implementing this challenging project at the field site.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public Health, Epidemiology, biostatistics and immunology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 09 Nov 21 |
|
|
Version 1 08 Jul 21 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)