Keywords
Tetracyclines, β-lactams, carbonylation, proteins, meat
This article is included in the Agriculture, Food and Nutrition gateway.
Tetracyclines, β-lactams, carbonylation, proteins, meat
Tetracyclines (TCs) and β-lactams (β-Lts) are widely used antibiotics in veterinary medicine for the treatment and prophylaxis of bacterial diseases affecting animals.1 In poultry, these antibiotics are employed to treat diseases caused by gram-positive and gram-negative bacteria, and in some cases are also administered at sub-therapeutic concentrations in the feed to promote the animal’s growth and ensure productive performance.2,3 In this context, this extensive and often indiscriminate use of TCs and β-LTs in poultry can induce the presence of their residues in poultry products such as chicken breast and eggs; these residues can reach and be ingested by consumers, and promote negative effects on their health, mainly through antimicrobial resistance.1
In order to protect to consumer, the European Commission and the Codex Alimentarius have stablished the maximum residue level (MRL) for antibiotics in products of animal origin. The MRL is defined as the highest level of residue of a pesticide or antibiotic that is legally tolerated in food and is not expected to cause harm to humans. Despite the regulation, several investigations have reported levels of TCs and β-LTs greater than their MRLs in chicken meat and other products derived from poultry farms, such as eggs; this could promote negative effects on consumers stated above.4,5
Despite the recurring presence of antibiotic residues in chicken meat and its derivatives, no investigations evaluating the effects of TCs and β-LTs at their MRLs on chicken meat components, including proteins, are being carried out, even though proteins are one of its most abundant constituents. In previous studies we have demonstrated that residues of these antibiotics at their MRLs are capable of inducing oxidative stress on biological models such as beef6 and milk,7 which could have a negative impact on food safety and quality. Protein carbonylation is currently recognized as one of the main biomarkers for oxidative stress in food,8 and several publications have demonstrated its impact in chicken meat and the alteration of its functional properties such as solubility and digestibility.9,10 In addition, the effects promoted by the consumption of foods with carbonylated proteins, such as metabolic disorders and inflammatory processes at the intestinal level and even the promotion of carcinogenic processes, have also been demonstrated.11,12
Chicken meat is the most common product consumed in the word due its affordability, although chicken meat proteins are susceptible to suffer oxidative reactions which can be promoted by external factors as animal feed, slaughter, processing and storage. Thus, the objective of this study is to evaluate the in vitro carbonylation induced by tetracyclines and β-lactams residues on chicken muscle proteins.
Primary standards of Ampicillin trihydrate (99.0%, AMP), Benzathine penicillin G tretrahydrate (97.2%, PNG), Dicloxacillin sodium hydrate (99.0%, DCL), Oxacillin salt hydrate (99.0%, OXA), Tetracycline hydrochloride (97.7%, TC), Doxycycline hyclate (98.7%, DXC), Chlortetracycline hydrochloride (94.6%, CTC) and Oxytetracycline hydrochloride (95.0%, OTC) were supplied by Dr. Ehrenstofer GmbH (Germany). The 2,4-Dinitrophenylhydrazine, potassium phosphate, sodium azide, hydrochloric acid, sodium bicarbonate and calcium chloride were purchased from PanReac (Barcelona, Spain). Analytical grade methanol, sodium hydroxide EMSURE (99%), biotechnological grade sodium chloride, 2-mercaptoethanol, and coomassie blue brilliant G250 (CBB) were purchased from Merck (Darmstadt, Germany). Bovine serum albumin (BSA) free of fatty acids (98%) was supplied from Sigma Aldrich (San Luis, USA). Water was purified with a Milli-Q system (Millipore, Bedford, MA, USA).
Individual stock solutions of tetracyclines and β-lactams (100 μg·mL-1) were prepared with milli-Q water and methanol, respectively. For the sample’s contamination with the antibiotics of interest, serial dilutions of the stocks were made until obtaining an antibiotic concentration of 10 μg·mL−1 (working solution), in order to avoid sample protein precipitation during the sample preparation process.13
Chicken breast samples were purchased from a local market in Cartagena, Colombia. They were stored in plastic polyethylene bags, transported to the laboratory maintaining cold chain (4°C), cut and cleaned to remove visible connective tissue and then, mechanically homogenized using a blender (Powergen by Fisher). Then, one-gram replicates of homogenized samples were collected in falcon tubes and stored at -20°C until further assays. This procedure was described by Marquez et al. (2020).13
Chicken breast aliquots were randomly divided in control samples and samples to contaminate with tetracyclines and β-lactams. Then, samples were contaminated individually with 200 μL of each antibiotic working solutions until reaching final concentrations of 0.5, 1.0 and 1.5 MRL (Table 1), followed by vortexing for 30s and then incubation for 1 h at room temperature in the dark.14 All assays were carried out with three replicates.
To obtain the sarcoplasmic, myofibrillar and insoluble proteins, the method developed by Marquez et al. (2020) was employed. Briefly, homogenized samples were mixed with 10 mL of low ionic strength buffer (0.05 M K3PO4, 1 mM NaN3, 2 mM EDTA, pH 7.3), then, vortexing at 3,000 rpm for 3.5 minutes and centrifugation at 11,150 xg 10 minutes and 1 °C were carried out. The supernatant (sarcoplasmic proteins) was collected and refrigerated, and the pellet was resuspended with 5 mL of low ionic strength buffer, centrifuged and the supernatant was unified with the sarcoplasmic proteins fraction initially obtained. The remaining pellet was resuspended with 5 mL of high ionic strength buffer (0.55 M KCl, 0.05 M K3PO4, 1mM NaN3, 2 mM EDTA, pH 7.3) and the same vortexing and centrifugation steps were realized.). The new supernatant (myofibrillar proteins) was collected and also kept refrigerated at 4°C. The final pellet was resuspended in 0.15 M KCl to obtain the insoluble proteins.13 Protein concentration was determined by the Bradford method (Coomassie blue brilliant/ethanol/phosphoric acid). For this, a calibration curve was realized using standard solutions of bovine serum albumin at concentrations between 0.0625 and 1.0 mg·L−1, which were put in microplates with the samples and the Braford reactive. Then, the absorbance was measured at 595 nm.15
Protein carbonyl content was measured according to Mesquita et al. (2014) with some modifications described by Marquez et al. (2020).13,16 For this, 300 μL of 2,4-dinitrophenylhydrazine (DNPH, 10 mM in 0.5M H3PO4) was added to the same volume of protein solution (150 μg) and incubated in the dark for 10 minutes. Next, 160 μL of this solution was placed in a 96-well plate and 40 μL of 6M NaOH were added and incubation for 10 minutes was carried out. The change of colour was measured at 450 nm (FLUOstar Omega spectrophotometer, BMG-Lab Tech). The protein carbonyl content was calculated using the DNPH molar extinction coefficient corrected for microplates (ε = 11154 μM-1.cm-1). Protein carbonylation was expressed as nmol of carbonyls·mg-1 of protein.
Values are reported as mean ± SEM of three independent determinations. To assess the effect of antibiotics concentration on protein carbonylation, a unifactorial ANOVA with three levels and blank at 95% confidence was performed (GraphPad Prism V5.01). Multiple comparisons of the means were made using the Tukey adjustment when the ANOVA was significant (p < 0.05) for control and contaminated samples.
The oxidative capability of the assayed antibiotics was evaluated by determination of the carbonylation degree of exposed sarcoplasmic, myofibrillar and insoluble chicken breast proteins, and was expressed as the carbonyl index (CI) in nmol of carbonyls·mg-1 of proteins. CIs found in the control samples were on average 7.40 ± 0.85; 8.67 ± 1.03 and 12.79 ± 0.5178 nmol of carbonyls·mg-1 of proteins for sarcoplasmic, myofibrillar and insoluble, respectively. When those values were compared with the treated samples, it was observed that 16 of the 24 treatments assayed (66.7%) significantly increased their carbonyl groups, as shown in Figure 1.
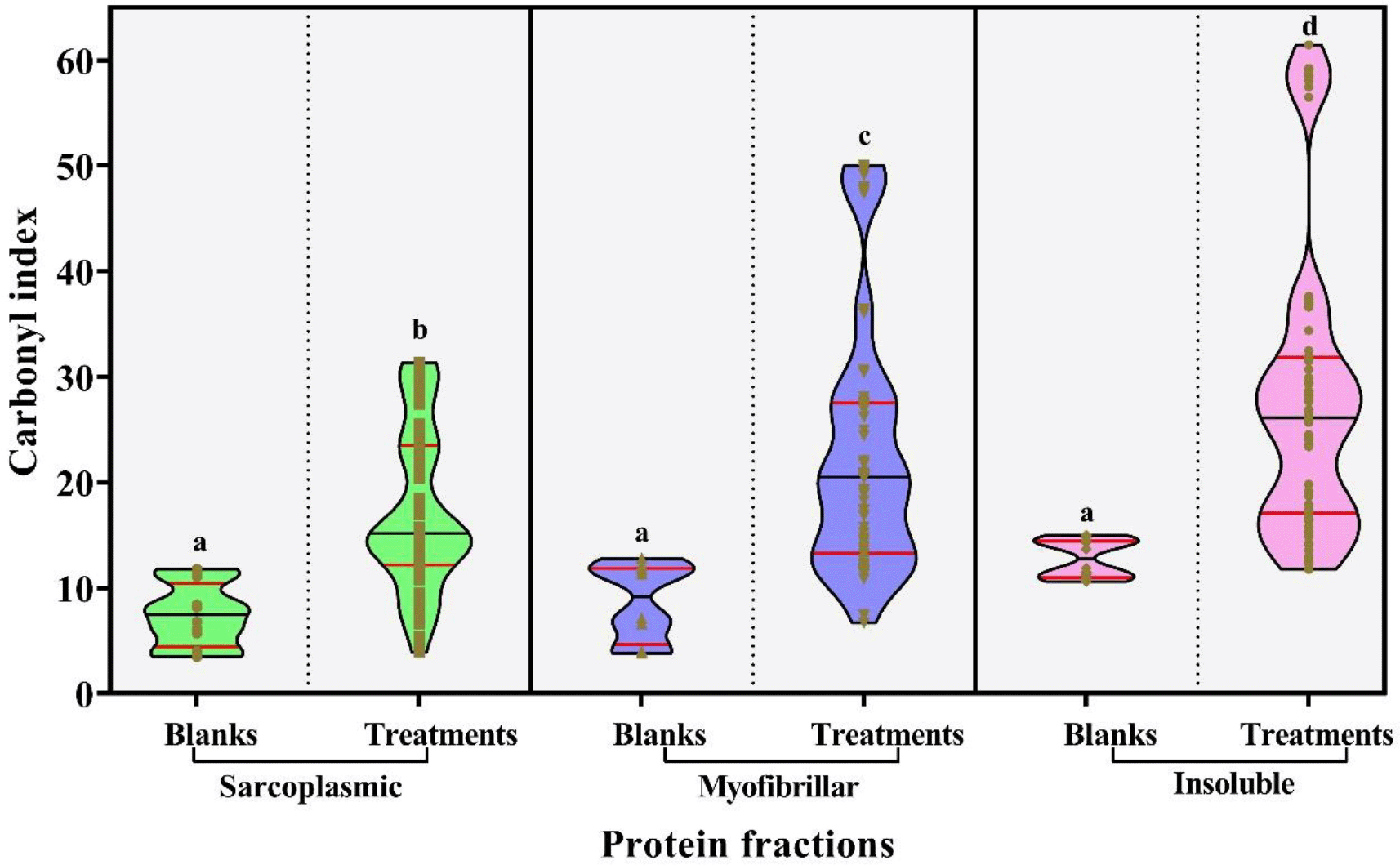
The colors of the violins allude to the type of protein fraction: Green: sarcoplasmic proteins; Blue: myofibrillar proteins and Purple: insoluble proteins. The medians and quartiles are showed as black and red lines in the violin boxes, respectively. Different letters on the plots expressed significant differences in protein carbonylation between samples.
The CIs induced by antibiotics on protein fractions were between 3.92 – 31.38; 6.72 – 50.07 and 11.77–61.49 nmol of carbonyls·mg-1 of proteins for sarcoplasmic, myofibrillar and insoluble, respectively. In addition, the developed Kruskal-Wallis test among the CIs from the three proteins groups showed that there were significant differences between their medians (p<0.05), the insoluble proteins being the ones that showed the highest CIs (28.04 ± 1.61 nmol of carbonyls·mg-1 of proteins), followed by myofibrillar and sarcoplasmic proteins with mean values of 23.07 ± 1.42 and 17.28 ± 0.89 nmol of carbonyls·mg-1 of proteins, respectively. Next, we described the behavior of induced carbonylation by each group of antibiotics.
TCs’ oxidative capability is shown in Figure 2. In sarcoplasmic proteins, all assayed concentrations of CTC, OTC and DXC induced a significant carbonylation compared to the control (p < 0.05); in contrast, the samples contaminated with TC, only showed a significant increase at 0.5 and 1.5 MRL (p < 0.05). The maximum oxidant power of TC, CTC and DXC was observed at 1.5 MRL, while OTC was set at 0.5 MRL. Contrarily, in myofibrillar proteins, TC, CTC and OTC promoted a significantly higher carbonylation compare to control (p < 0.05) at all concentrations assayed. DXC only induced a significant carbonylation at 1.0 MRL regarding to the control (p < 0.05). Unlike to sarcoplasmic proteins, the maximum oxidant power of TC, OTC and DXC was observed at 1.0 MRL, while CTC’s continued at 1.5 MRL (p < 0.05).
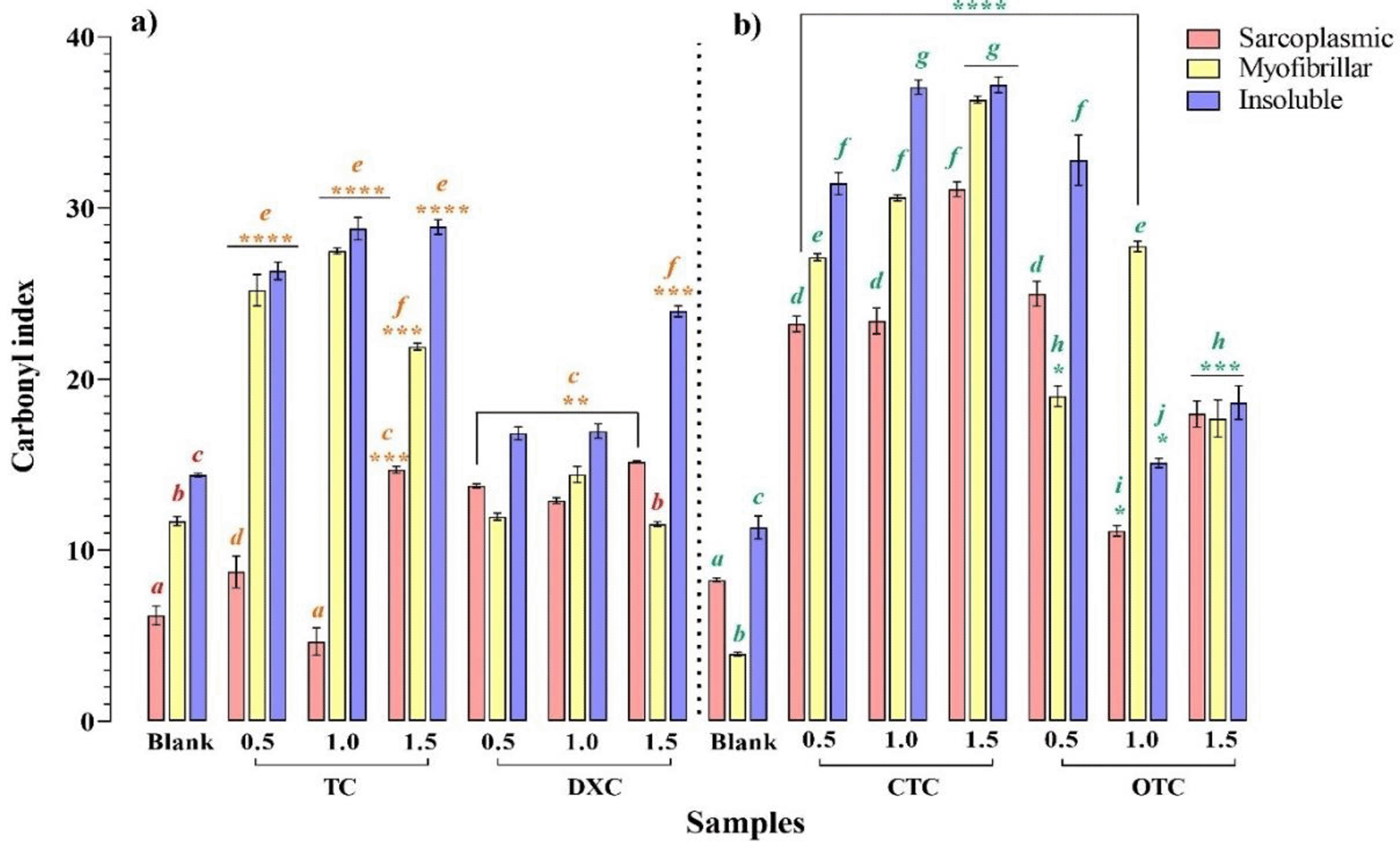
The columns in the graph are grouped as: a) TC and DXC treatment´s and their blanks b) CTC and OTC and their blanks. Mean values ± SEM. a-j Different letters on the bars denote statistically significant differences. Means having different superscripts differ between blank and tetracyclines concentration (p < 0.05). ****p < 0.0001, ***p < 0.005, **p < 0.05 denote statistically significant differences among treatment and their respective control.
Finally, for insoluble proteins, all concentrations of tetracyclines induced significant carbonylation with respect to control (p < 0.05). The maximum carbonylation promoted by TC and CTC was observed at 1.0 and 1.5 MRL (p > 0.05), while OTC and DXC were at 0.5 and 1.5 MRL, respectively.
β-Lts-induced carbonylation is shown in Figure 3. In sarcoplasmic proteins, all the assayed concentrations induced significant carbonylation on contaminated samples compared to control (p < 0.05). For samples contaminated with AMP and PNG, maximum carbonylation was observed at 1.5 MRL, while in samples contaminated with DCL, maximum carbonylation was set at 1.0 MRL;for OXA the values were set at 0.5 and 1.0 MRL. There were no statistical differences between their CIs (p > 0.05). In contrast, in myofibrillar proteins, DCL and OXA induced the same carbonylation in treated samples at all assayed concentrations (p > 0.05), while AMP only induced a significant carbonylation at 0.5 and 1.5 MRL with respect to control (p < 0.05), and PNG a 1.5 MRL carbonylation. Finally, for insoluble proteins, PNG, DCL and OXA promoted a significantly higher carbonylation than control at all concentrations assayed (p < 0.05), while AMP only led to a 1.5 MRL carbonylation. For AMP and OXA, the maximum oxidant power was induced at 1.5 MRL, whereas that of DCL was similar for all three concentrations (p > 0.05).
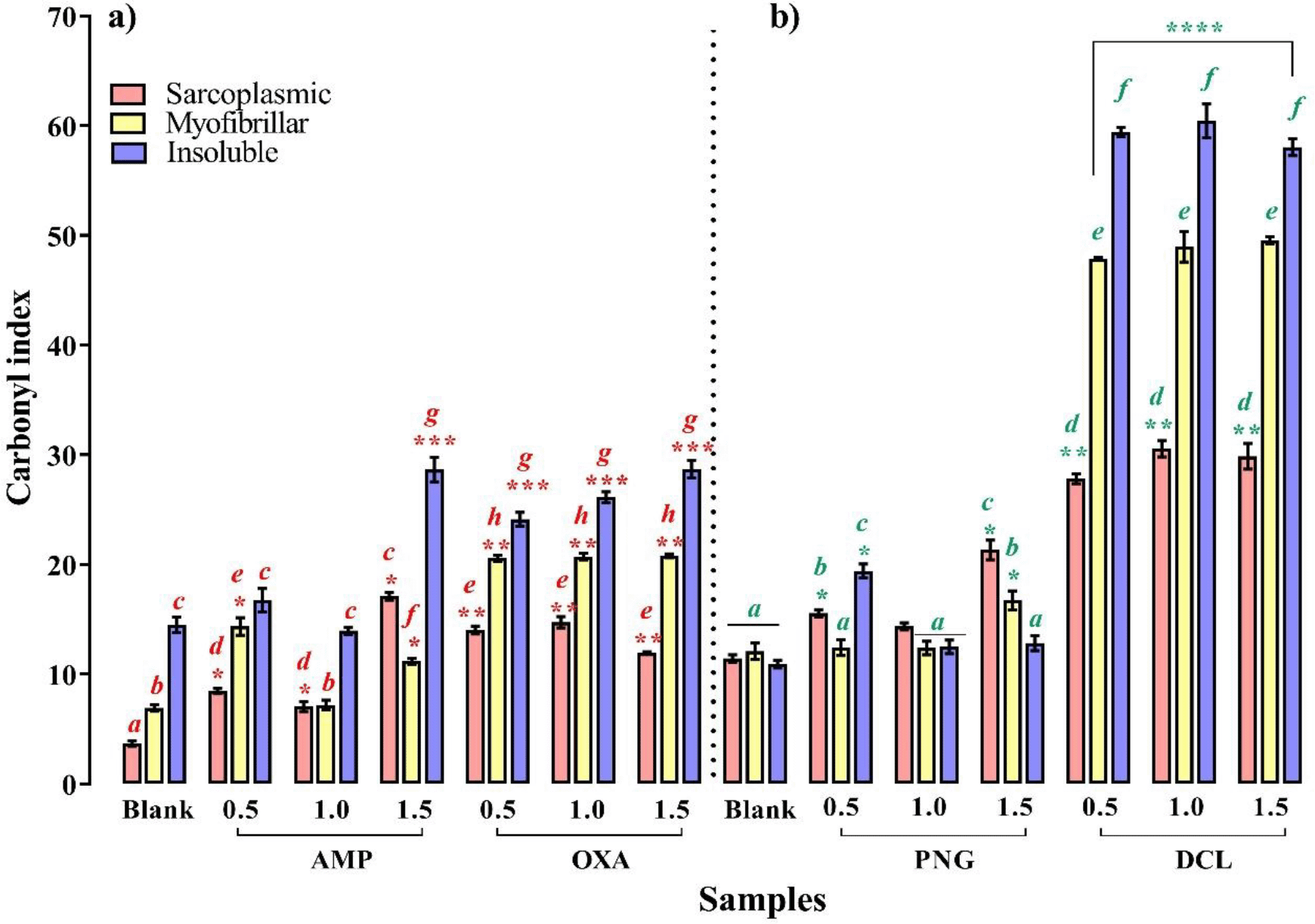
Mean values ± SEM. Letters a-j above the bars denote statistically significant differences in carbonylation compared to blank. Means having different superscripts differ between blank and tetracyclines concentration (p < 0.05). ****p < 0.0001, ***p < 0.005, **p < 0.05 denote statistically significant differences among treatment and their respective control.
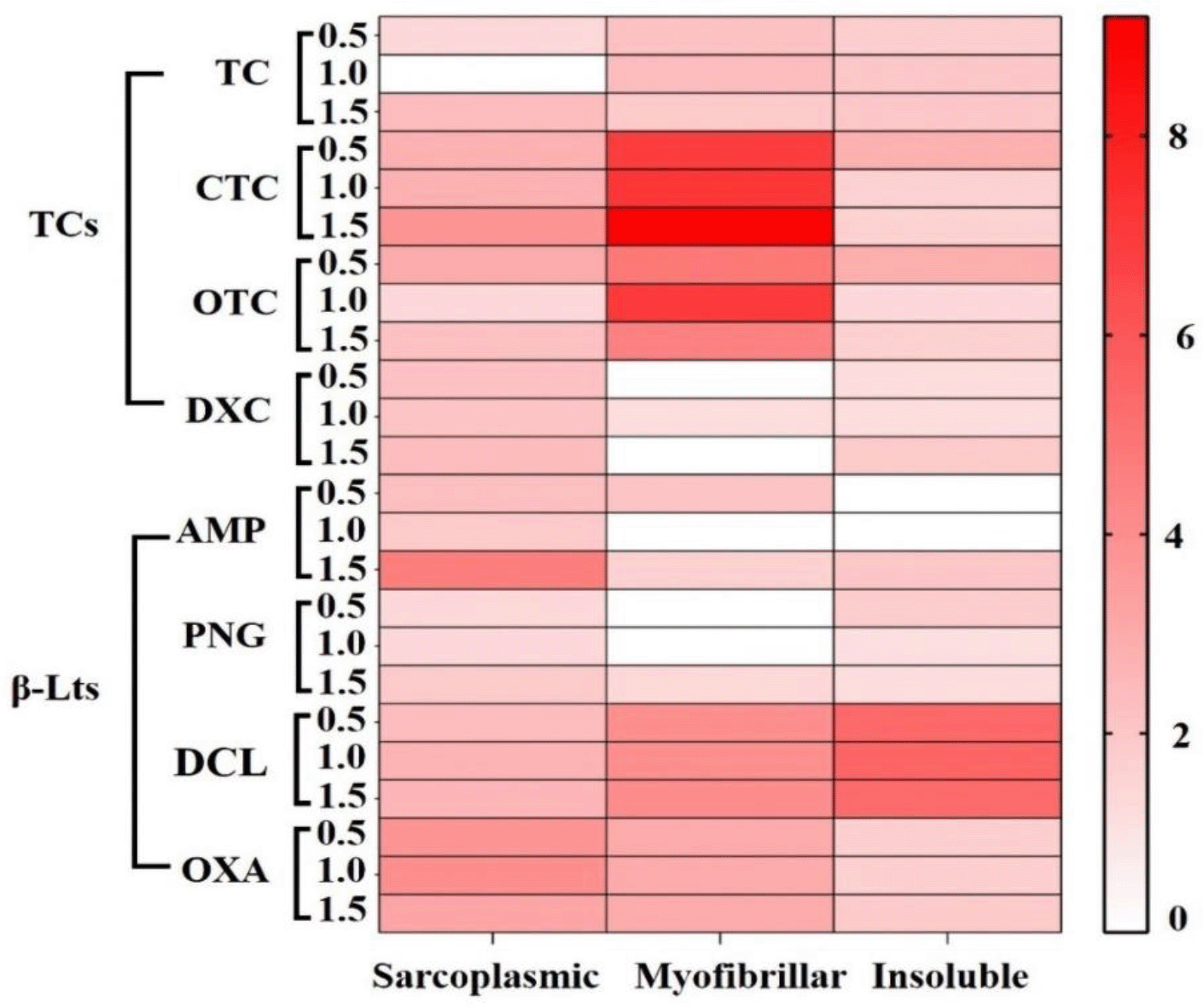
Figure 4 shows the oxidant power of TCs and β-LTs on sarcoplasmic, myofibrillar and insoluble proteins from chicken breast; oxidant power was calculated using the ratio between the CIs from treated samples and from the controls (Carbonyl index of protein fraction from contaminated samples/Carbonyl index of blank samples). As showed, among all antibiotics assayed, CTC and OTC promoted the highest carbonylation in myofibrillar proteins at all assayed concentrations (with average ratios of 7.9 and 5.4, respectively); these were followed by DCL in the insoluble (average ratio of 5.4) and myofibrillar fractions (average ratio of 4.0) and OXA in the sarcoplasmic and myofibrillar fractions (average ratios of 3.7 and 3 respectively). Oppositely, DXC, AMP and PNG induced the lowest carbonylation, mainly in myofibrillar (average ratios of 0.4, 0.5 and 0.5, respectively) and insoluble fractions (average ratios of 1.4, 0.7 and 1.4, respectively).
The results of the comparative analysis between the carbonylation induced by TCs and β-Lts according to the type of fraction is described in the first item (Figure 1). On the other hand, the medians of the carbonylation indices (CIs) promoted by the two groups of antibiotics evaluated through the Mann-Whitney test were compared; results showed that there were no significant differences between the CIs in the samples exposed to both groups of antibiotics (p = 0.3714), Figure 5.
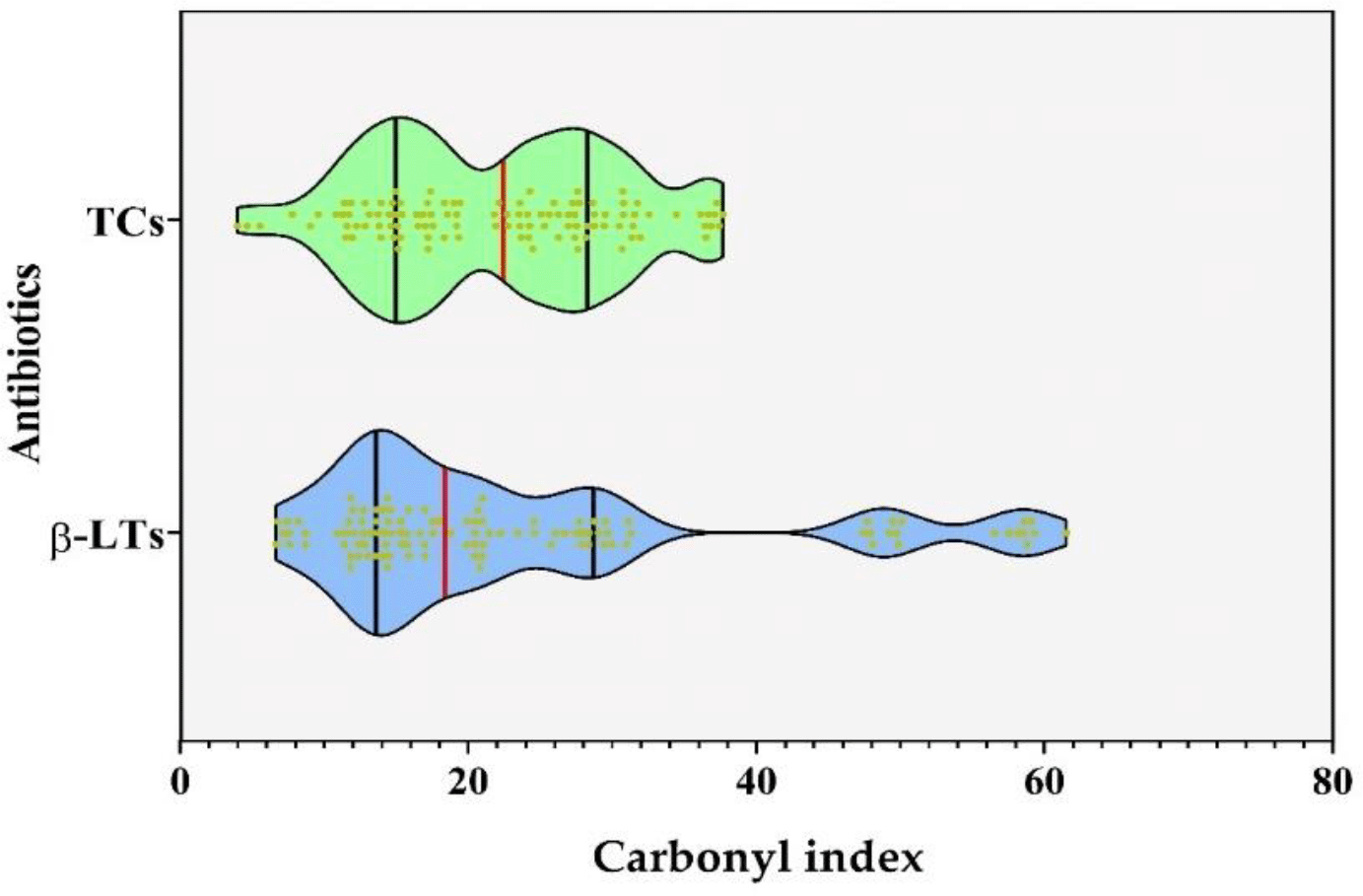
The medians and quartiles are showed as black and red lines in the violin boxes, respectively.
According to the carbonylation variability induced by the assayed antibiotics, we decided to assess individual and multiple correlations among CIs and independent variables assessed in the study: protein fraction, type of antibiotic, evaluated concentration of each antibiotic, and octanol-water partition coefficient (LogP). This last variable was included given that the antibiotics evaluated, by belonging to the same group, have similar chemical structures (pharmacophore) differing only in their radicals; therefore one of the most used parameters to evaluate differences in their toxicological behaviors in in vitro models are their solubility variations (LogP).17
Simple regression analysis results between CIs induced by antibiotics at their respective concentration, and LogP revealed a significantly positive relationship for both variables combinations (R2 = 0.0327, p = 0.0044 for CIs and antibiotics concentrations, and R2 = 0.1235, p = 0.0019, for CIs and LogP). This indicates that they represent important parameters in the oxidant properties of antibiotics (Figure 6a and 6b). When comparing adjusted determination coefficients obtained in correlation analyses, we were able to establish a hierarchy in the effect exercised by the evaluated independent variables (concentration and LogP) on the antibiotics-induced carbonylation, showing the following increasing order: LogP > concentration. Nevertheless, in order to evaluate the joint correlation level of independent variables assayed (concentration and LogP) vs the antibiotic-induced carbonylation, a multiple linear regression analysis was performed. Thus, the comparison between adjusted R2 obtained in the simple and multiple linear regression analysis showed that R2 in combined variables (14.48%) was higher than that of individual ones (3.27% and 12.35% for concentration and LogP, respectively), thus attributing the antibiotic-induced carbonylation effect to the synergic effect of the evaluated independent variables (p < 0.0001).
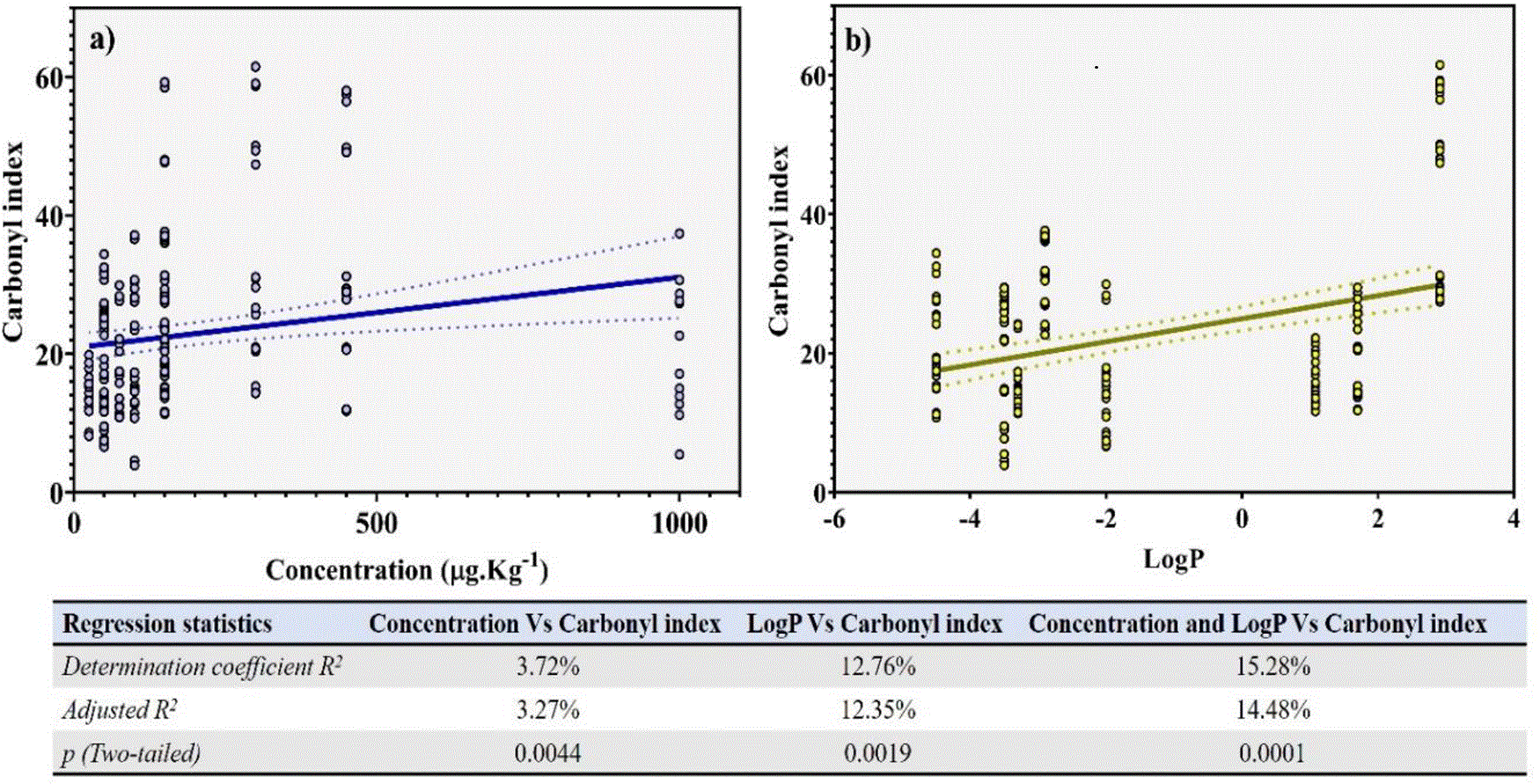
These parameters were used to evaluate the variables’ relation level were the correlation and determination coefficients.
The results described before show the capability of TCs and β-LTs to promote the carbonylation of chicken breast proteins even at concentrations under their MRLs.
In addition, when comparing the carbonylation induced by tetracyclines in three different protein fractions, it can be observed that sarcoplasmic and myofibrillar proteins were the most oxidized ones (Figure 4). This behavior could be associated to accessibility of proteins in muscle cells: sarcoplasmic being more available than myofibrillar, which are more available than insoluble proteins; this is due to sarcoplasmic proteins being soluble in sarcolemma, while myofibrillar and insoluble proteins are stored in myofibrilla and connective tissue, respectively.13,18
Finally, carbonylation of chicken breast proteins provides evidence for the ability of tetracyclines to promote oxidative stress, which is consistent with assays carried out with several biological models.19–24 According to Wen et al. (2012), CTC induced oxidative stress in maize root through the production of hydroxyl radicals19; Pes et al. (2018) reported a decrease of antioxidant enzymes in fish when these were exposed to OTC; Hang et al. (2019) showed that exposure to TC induced oxidative stress in ryegrass seedlings mediated by the increase of lipid oxidation and decrease of antioxidants enzymes.9 However, according to the literature, DXC has antioxidant properties, which is contradictory to our results.21–23 This fact is interesting since the concentrations assayed in this study were lower than the ones used in studies where the protecting effect against oxidation was observed (16 times approximately)21–23; maybe this behavior could be associated to endocrine-disrupting effects.
In a similar way as tetracyclines, these results show the capacity of β-lactams to induce oxidation in chicken breast proteins even at levels under their MRLs, and therefore their ability to induce oxidative stress. However, these results are contradictory to the literature, because β-lactams have been attributed antioxidant properties: Berczyński et al. (2017) demonstrated the antioxidant capacity of β-lactams in the following descending order: ampicillin, penicillin, dicloxacillin and finally oxacillin, against reactive oxygen species.24 Although Dwyer et al. (2014), observed that some antibiotics, suchas ampicillin induced oxidative stress to increase their antibiotic lethality. Thus, β-lactam behavior could be bivalent (oxidant/antioxidant), explaining the lower levels of carbonylation induced of chicken breast proteins (Figure 4). While comparing the carbonylation on three protein fractions, only DCL and OXA promoted greater oxidative damage on these (Figure 4), probably mediated by a largest number of molecules of DCL and OXA available in the muscle cell, these amounting to 6 times the concentrations of PGN and AMP.4,13
According to the literature, the carbonylation induced by residues of tetracyclines and β-lactams at MRL concentrations are similar to those promoted by fluroquinolones MRLs on beef proteins, which was reported by Marquez et al. (2020). In this study, fluoroquinolones also induced carbonylation even at concentrations under MRL values, and related to solubility loss and decreased protein digestibility.13 Therefore, it would be expected that these types of changes can occur in chicken muscle proteins as well and influence the meat’ nutritional value.14
In conclusion, our results showed the oxidizing effect of tetracycline and β -lactam residues on chicken breast proteins, even at concentrations considered safe. This causes concern because carbonylation continues to occur even after the animal is slaughtered, which means that under in vivo conditions protein oxidation may be greater. This could also affect the nutritional value of meat proteins, since carbonylation causes solubility loss and decreased digestibility in proteins, as well as loss of protein functionality.
Figshare: Raw data of chicken breast proteins exposure to antibiotics, https://doi.org/10.6084/m9.figshare.14799147.v3
This project contains the following underlying data:
• Data set 1: Raw data from carbonyl assay of tetracyclines.
• Data set 2: Raw data from carbonyl assay of β-lactams.
• Raw data Figures.
• Raw data Figures from GraphPad Prism.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors express their gratitude to the University of Cartagena for grants 078 and 095/2019 and Corporación Universitaria Rafael Núñez.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
A. Marrugo-Padilla thanks Colciencias for the scholarships National Doctorates No 785-1047457972. The authors would like to thank Judith Rodriguez Cavallo for her technical assistance and María Alejandra Mendez for editing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Meat science, protein oxidation, peptide chemistry, omics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Poultry meat quality
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Jul 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)