Keywords
Preeclampsia, Correlation, Onset, Severity, Ghana
Preeclampsia, Correlation, Onset, Severity, Ghana
ALT, alanine aminotransferase
AST, aspartate aminotransferase
CRT, creatinine
ELISA, enzyme-linked immunosorbent assay
EOPE, early onset preeclampsia
ET-1, endothelin-1
GGT, gamma glutamyl transferase
HDL, high density lipoprotein
LDL, low density lipoprotein
LOPE, late onset preeclampsia
PE, preeclampsia
PNS, preeclampsia without severe feature
PS, preeclampsia with severe features
TCHOL, total cholesterol
TRIG, triglycerides
sNGAL, serum neutrophil gelatinase-associated lipocalin
This study sought to determine the correlation between the onset and severity of preeclampsia (PE) and maternal variables in a Ghanaian population. PE is a multi-system disorder of pregnancy with unknown aetiology. It is responsible for about 3–8% of the global burden of maternal and neonatal morbidity and mortality.1 Hypertension, with or without proteinuria, occurring after 20 weeks of gestation, is the cardinal symptom of PE. The occurrence of PE may be early, happening before 34 weeks (EOPE) or late (LOPE). Preeclampsia may also be characterized by severe features (PS) or without severe features (PNS).2
Although the aetiology of PE is poorly understood, aberrant trophoblastic invasion of the placenta, placental hypoxia and endothelial dysfunction have been suggested. The generalized inflammatory process, angiogenic imbalance, endothelial injury and increased oxidative stress in PE, may cause organ and organ–system dysfunctions including the renal, the hepatic and the cardiovascular systems.2,3
Previous studies have demonstrated that maternal lipids, liver function, renal function, ET-1 and sNGAL are affected by the onset and severity of PE.4,5 However, the influence of genetic and environmental factors on PE may lead to population and sub-population variabilities in the correlations between the onset and severity of PE and maternal variables.6,7 There is therefore the need for population-specific studies to establish local reference data for the early diagnosis and management of PE.
This was a case–control study from January to June 2018. The study was conducted at the Richard Novarti Catholic Hospital, located at Sogakope in the South Tongu District of the Volta Region of Ghana.
The study involved 270 pregnant women aged 18–37 years. The sample size was calculated following the recommendations of Fahim, Negida.9 The study population was divided into two groups: 135 women with PE who were further stratified based on the onset of PE as either EOPE (n = 71) or LOPE (n = 64) and also based on the presence or the absence of severe features as either PNS (n = 87) or PS (n = 48). The cases were matched by maternal and gestational age to 135 women with normotensive and uncomplicated pregnancies at sampling (serving as controls). The inclusion criterion was a diagnosis of PE, whether early or late onset, with or without severe features. Exclusion criteria included: all women with twin gestations; those who were previously diagnosed with chronic hypertension, sickle cell anaemia, diabetes mellitus, gestational diabetes, cardiovascular disorder, renal disease and those who were on antihypertensive or magnesium medication before the recruitment. Sociodemographic and clinical information was collected using a structured questionnaire and also from their medical records.
The definition of PE, its onset and severity was based on the recommendations of the American College of Obstetricians and Gynecologists.2 Preeclampsia was defined as the new-onset of hypertension (systolic ≥140 mmHg, diastolic ≥90 mmHg, taken 4 hours apart) with proteinuria (2+ on the dipstick at presentation) after 20 weeks of gestation. Early-onset PE was defined as PE occurring before 34 weeks of gestation and LOPE was defined as PE occurring at/after 34 weeks of gestation. Preeclampsia with severe features was defined as PE characterized by systolic (≥160 mmHg) or diastolic (≥110 mmHg ) blood pressure while PNS was defined as PE without severe features.
Blood pressure was measured with the aid of a cuff sphygmomanometer according to the fifth Korotkoff sound. Urine screening for proteinuria was performed using a urine dipstick. A single peripheral venous blood sample was collected from each subject’s antecubital vein after an overnight fast (12 h) into an EDTA and a gel-separator tube. The serum samples were allowed to clot for 30 min at 4°C and both tubes were then centrifuged at 1500 rpm for 10 min to separate the serum/plasma. Serum and plasma samples were aliquoted into plastic cryotubes and then frozen at −25oC until analysis. Serum NGAL was analysed in duplicates using the ELISA kit from abcam (152 Grove Street Waltham, MA 02453., USA). The kit had a sensitivity of 14.8 pg/mL, within a linearity range of 46.9 pg/mL - 3000 pg/mL. The intra-assay and inter-assay coefficients of variation were ≤3.8% and ≤2.7% respectively. Serum ET-1 was analyzed in duplicates using Human ELISA kit (Biomedica Medizinprodukte GmbH, Divischgasse 4, 1210 Vienna, Austria). The sensitivity of the ET-1 ELISA kit was 0.086 pg/mL within a linearity range of 0.00–12.85 pg/mL. The intra-assay and the inter-assay coefficients of variation were ≤4% and ≤5% respectively. The TCHOL, HDL, LDL, TRIG, AST, ALT, GGT and CRT were assayed using the plasma/serum samples (as appropriate) through routine biochemistry analysis on the BT 1500 automated biochemistry analyzer (Biotechnica Instruments, SPA, Italy) following the manufacturer’s instructions and using the recommended reagents. The samples in this study were not previously thawed and refrozen.
Data were entered into a Microsoft Excel (RRID:SCR_016137) spreadsheet (Google Sheets (RRID:SCR_017679) is an open access alternative) and statistical analysis was performed using SPSS (v23) (RRID:SCR_019096), and GraphPad Prism (v8) (RRID:SCR_002798); JASP (RRID:SCR_015823) is an open access alternative. The normality of the data was checked using the Kolmogorov–Smirnov test. Continuous variables were reported as median (IQR) or mean ± SD (as appropriate) and number (%) for categorical variables. Differences between median and mean values were determined using the Mann–Whitney U test and the student t-test respectively. Spearman rank correlation and linear regression analysis were used to determine the relationship between the independent and the outcome variables. A probability level of P < 0.050 was considered statistically significant.
The general characteristics of the study population are summarized in Table 1. This was an observational study with only one stage and as such there were no loss of participants. The controls and the cases (PE) did not differ in maternal and gestational age (P > 0.050). The BMI, sNGAL, ET-1, TCHOL, LDL, TRIG, ALT, AST and CRT were significantly elevated while HDL was markedly reduced in PE as compared to the controls (P < 0.010). Also, the maternal and gestational age, BMI, GGT and CRT were significantly elevated in LOPE as compared to EOPE (P < 0.050). In comparing PNS to PS, maternal age was reduced while sNGAL, ET-1, TRIG, ALT, AST and GGT were considerably increased in PS (P < 0.050). The correlation and linear regression analysis between the onset of PE and maternal variables are shown in Figure 1. Aside from maternal age and GGT that showed no significant correlation with the onset of PE, HDL was inversely correlated (r = −0.125, P = 0.048), while, blood pressure, BMI, parity and proteinuria were all positively correlated with the onset of PE (P < 0.050). The correlation and linear regression analysis between the severity of PE and maternal variables are shown in Figure 2. Maternal HDL was inversely correlated (r = −0.199, P = 0.001), while the rest of the maternal variables (except maternal age, parity and GGT) were all positively correlated with the severity of PE (P < 0.050).
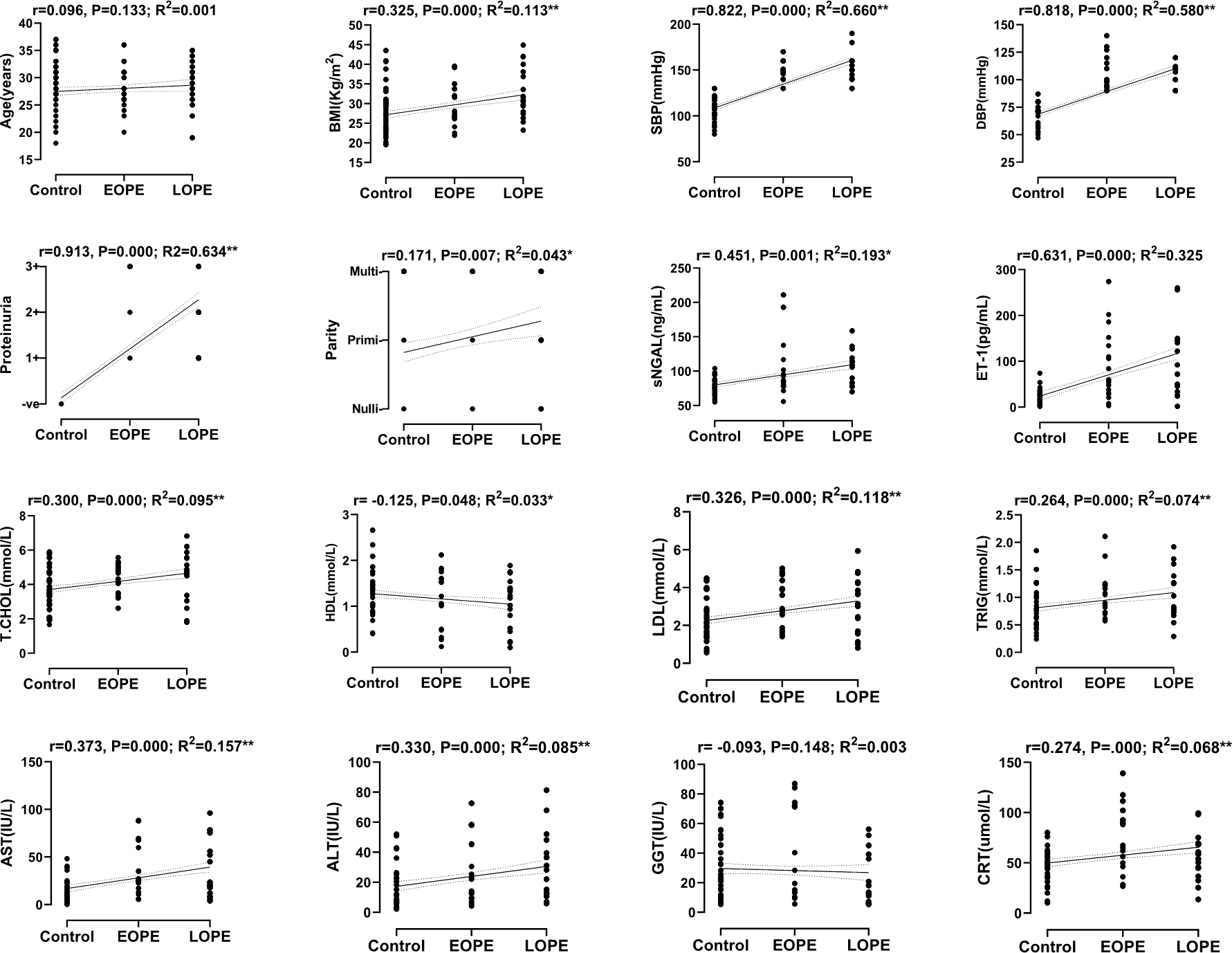
The control, EOPE and LOPE were dummy coded before linear regression and correlation analysis.
*Significant at the 0.010 level, **Significant at the 0.001 level.
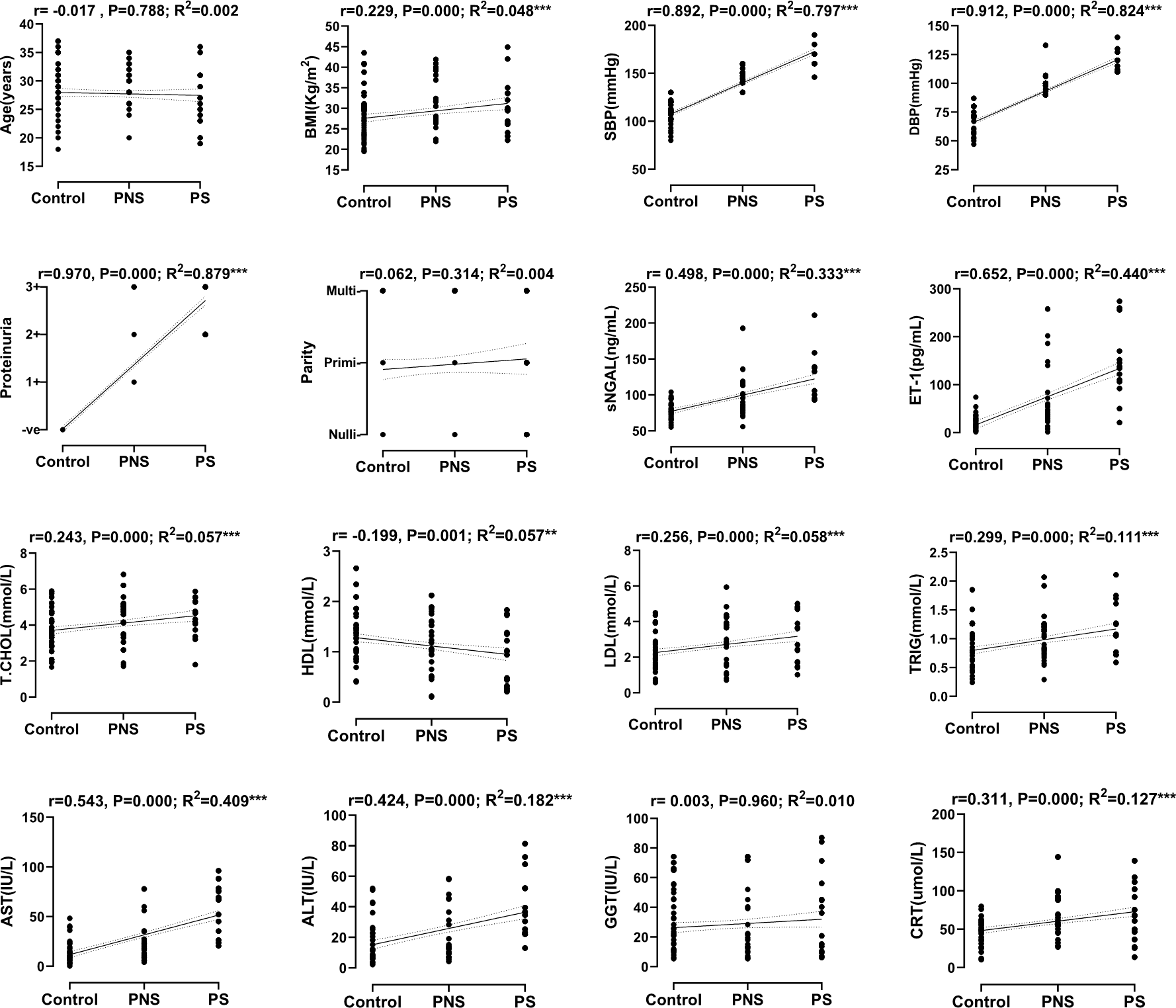
The control, PNS and PS were dummy coded before linear regression and correlation analysis.
*Significant at the 0.010 level, **Significant at the 0.001 level.
| Variable | SI unit | Control | PE | Onset | Severity | ||
|---|---|---|---|---|---|---|---|
| n (135) | n (135) | EOPE n (71) | LOPE n (64) | PNS n (87) | PS n (48) | ||
| Socio-demographics and anthropometrics | |||||||
| Age | years | 27.8 ± 4.63 | 27.9 ± 4.40 | 27.0 ± 4.04 | 29.2 ± 4.12†† | 28.5 ± 3.78 | 26.7 ± 4.84‡ |
| GA | weeks | 29.7 ± 3.02 | 29.9 ± 3.00 | 28.0 ± 2.10 | 32.4 ± 2.50 ††† | 30.1 ± 3.15 | 29.7 ± 3.04 |
| BMI | kg/m2 | 27.4 ± 5.83 | 30.2 ± 6.14 ** | 28.5 ± 5.03 | 32.9 ± 6.24 ††† | 30.0 ± 6.15 | 30.6 ± 6.38 |
| SBP | mmHg | 105.4(11.91) | 151.8 ± 12.24** | 151.4 ± 9.17 | 152.3 ± 15.30 | 146.7 ± 8.39 | 164.7 ± 11.09‡‡ |
| DBP | mmHg | 65.0(9.25) | 103.6 ± 12.83** | 105.3 ± 14.04 | 101.1 ± 10.57 | 95.6 ± 5.47 | 115.3 ± 6.77‡‡ |
| Proteinuria | |||||||
| Neg | - | 135(100) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| Pos (1+) | - | 0(0.0) | 60(44.4) | 32(45.0) | 25(38.9) | 54(62.1) | 0(0.0) |
| Pos (2+) | - | 0(0.0) | 27(20.0) | 21(30.0) | 18(27.8) | 30(34.5) | 16(33.3) |
| Pos (3+) | - | 0(0.0) | 48(35.6) | 18(25.0) | 21(33.3) | 3(3.4) | 32(66.7) |
| Parity | |||||||
| Nulliparous | - | 60(44.4) | 48(35.6) | 43(60.0) | 7(11.1) | 33(37.9) | 16(33.3) |
| Primiparous | - | 27(20.0) | 36(26.7) | 11(15.0) | 18(27.8) | 24(27.6) | 13(26.7) |
| Multiparous | - | 48(35.6) | 51(37.8) | 17(25.0) | 39(61.1) | 30(34.5) | 19(40.0) |
| Biomarkers | |||||||
| sNGAL | ng/mL | 79.1(72.6-85.4) | 95.2(78.6-116.9) ** | 84.9(78.6-101.7) | 108.8(82.5-119.1) | 84.3(77.3-113.0) | 132.5(100.2-139.1) ‡‡ |
| ET-1 | pg/mL | 17.9(10.0-27.8) | 60.0(30.0-146.0) ** | 59.0(30.0-134.0) | 82.0(46.0-148.0) | 44.0(24.5-72.0) | 136.0(106.0-170.0) ‡‡ |
| TCHOL | mmol/L | 3.5(2.8-4.7) | 4.6(3.5 ± 5.1) ** | 4.2(3.6-5.1) | 4.7(3.4-5.6) | 4.6(3.3-5.1) | 4.7(3.7-5.3) |
| HDL | mmol/L | 1.3(1.0-1.5) | 1.0(0.5-1.5) * | 1.1(0.6-1.6) | 1.2(0.5-1.4) | 1.1(0.7-1.5) | 1.0(0.3-1.4) |
| LDL | mmol/L | 2.1(1.5-2.7) | 2.8(1.7-3.9) ** | 2.8(2.1-3.9) | 3.4(1.7-4.3) | 2.8(1.7-3.9) | 2.7(1.7-4.7) |
| TRIG | mmol/L | 0.8(0.5-1.1) | 0.9(0.7-1.3) ** | 1.0(0.7-1.2) | 0.9(0.7-1.4) | 0.9(0.7-1.2) | 1.1(0.7-1.6) ‡ |
| ALT | IU/L | 11.1(6.2-22.5) | 23.7(16.3-56.1) ** | 25.7(14.2-53.8) | 21.8(12.2-56.1) | 19.3(10.4-25.1) | 67.9(35.4-78.5) ‡‡ |
| AST | IU/L | 11.2(6.1-23.0) | 22.1(10.6-45.0) ** | 18.1(9.2-45.1) | 25.2(12.5-39.5) | 12.7(9.2-35.2) | 35.6(23.1-52.3) ‡‡ |
| GGT | IU/L | 21.4(11.3-44.0) | 16.7(10.6-43.7) | 19.5(10.4-71.9) | 15.8(7.0-39.2) † | 14.4(10.9-36.5) | 36.2(10.3-56.2) ‡ |
| CRT | μmmol/L | 48.1(36.6-56.0) | 61.3(44.9-91.3) ** | 65.0(46.3-92.7) | 56.3(44.9-69.5) † | 60.2(45.1-89.0) | 67.4(36.6-102.1) |
The study aimed to determine the correlation between the onset and severity of PE and maternal variables. Maternal variables including lipids, liver enzymes (except GGT), creatinine, sNGAL, ET-1 were significantly correlated with the onset and severity of PE. Also, both systolic and diastolic blood pressure, proteinuria, and BMI were positively correlated with the onset and severity of PE.
The increased blood pressure in PE stems from the resistance in the vascular system and decreased intravascular volumes and cardiac output. There is a decrease in conduit artery compliance as well as the obliteration of the fall of nocturnal blood pressure. Hypertension in PE may contribute to organ damage including, acute kidney injury and renal dysfunction.10 Previous studies have shown that renal blood flow and glomerular filtration rates are decreased in PE as shown by the loss of podocytes, endothelial swelling and glomerular endotheliosis in biopsies from PE patients. Damaged endothelial cells in PE may induce clotting, as well as loss of anticoagulant ability as levels of nitric oxide and prostaglandin, plummet leading to kidney thrombotic microangiopathy.11,12 The associated renal dysfunction in PE may account for the elevated serum CRT and sNGAL. Serum NGAL is usually secreted directly into damaged renal tubular cells with the aim of re-epithelialization and has been shown to indicate renal injury much earlier than CRT. Serum NGAL is also involved in immune-system modulation as its levels increase in PE due to the generalized inflammation. The onset and severity of PE are also significantly correlated with proteinuria. Increased proteinuria in PE is due to increased permeability of the renal tubules partly due to the glomerular endotheliosis.10
PE is also associated with hepatic dysfunction and abnormal lipid metabolism as shown by elevation in some serum liver enzymes and lipids. This may be due to endothelial injury which causes hepatocellular necrosis and hepatic microcirculatory deterioration. Hepatic dysfunction may affect the synthetic functions of the liver including abnormalities in blood coagulation.11,13 It is has been shown that particles of HDL and LDL are remodelled during normal pregnancy becoming smaller and denser with increased proatherogenic potential and this is worsened by PE.14 Although the exact mechanism is unknown, the changes in maternal lipid metabolism in PE has been suggested to be a compensatory process to increase energy supply to the developing foetus.15
Serum levels of ET-1 were elevated in PE as compared to the controls and the levels increased with the severity of the symptoms of the disease.4,16–21 Several studies have demonstrated the role of ET-1 in the aetio-pathology of PE including the elevated circulating ET-1, increase in ET-1 converting enzyme (ECE) activity, the differential effect of systemic ECE inhibition and increased localized ET-1 production in tissues of maternal origin.16–18,22–24 Further evidence of the role of ET-1 in PE was the increased expression of markers of oxidative stress when placental tissue explant from normal pregnancy were incubated with exogenous ET-1 in-vitro. Also, ET-1 reduced JEG-3 cell lines proliferation, evidence of the possible role of ET-1 in preventing trophoblast invasion of the placenta in the early stages of PE.25 In the reduced uterine perfusion pressure (RUPP) model, where blood flow to the uterus was partially restricted, inducing placental hypoxia and ischemia, there was increased expression of preproendothelin mRNA, with the associated proteinuria, renal injury and endothelial dysfunction.26,27 However, issues have been raised regarding the interpretation of serum ET-1 results; firstly, ET-1 from syncytiotrophoblastic cells in the placenta would have been diluted by the time blood reaches the antecubital area for sampling, secondly, ET-1 is an autocrine/paracrine product whose serum levels does not reflect local tissue production. It has been suggested that an ET-1 receptor saturation study be performed, in addition to serum ET-1 estimation, to measure ET-1 receptor activation since a prolonged steady-state of ET-1 receptor saturation may be reached in PE through the continuous infusion of ET-1 from the uterine veins.28 This study is among a few studies coming from Ghana to have simultaneously determined the correlation between the onset and severity of PE with multiple maternal variables, including sNGAL and ET-1. However, the authors acknowledge some limitations; blood samples were collected after the manifestation of symptoms and diagnosis of preeclampsia. It is recommended that sampling is done in each trimester through to the post-partum period to determine the exact period changes in maternal variables begin to occur due to PE. Also, quantitative 24-hour urine protein or creatinine-to-protein ratio is preferred to the dipstick method in screening for proteinuria.
In conclusion, the onset and severity of PE are significantly correlated with maternal variables such as parity, proteinuria, blood pressure, lipids, liver enzymes, sNGAL, ET-1 and creatinine. There is therefore the need for regular monitoring of these maternal variables for the early detection and management of PE in the Ghanaian population.
OSF: Underlying data for ‘Correlation between maternal variables and the onset and severity of preeclampsia’. https://doi.org/10.17605/OSF.IO/CF8XB.
The project contains the following underlying data: maternal socio-demographics and anthropometric variables as well as serum/plasma fasting lipids, liver enzymes, creatinine, neutrophil gelatinase-associated lipocalin, and endothelin-1. The cases were grouped by the onset of PE and also by its severity.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
NA conceived the idea and designed the experiment, SJA performed the experiment and collected the data, MB analysed the data and wrote the manuscript. All authors provided critical feedback, read and approved the final manuscript.
All procedures performed in this study involving human participants were done following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of the University for Development Studies (UDS), Tamale.
Informed consent was obtained from all individual participants included in the study.
The authors will like to acknowledge the staff at the ante-natal clinic of the Novarti Catholic Hospital for helping in sample selection and data collection. We also acknowledge the staff of the Department of Biomedical Laboratory Science of the University for Development Studies for supervising the work. Lastly, we will like to thank all the participants for consenting to the study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222.Obstet Gynecol. 2020; 135 (6): e237-e260 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Maternal medicine
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Roberts JM, Hubel CA: The two stage model of preeclampsia: variations on the theme.Placenta. 2009; 30 Suppl A: S32-7 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 21 Jul 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)