Keywords
Extraction, Oil, Solvents, Supercritical Fluids, Ethanol and CO2
Extraction, Oil, Solvents, Supercritical Fluids, Ethanol and CO2
For Reddy (2019) and Tongnuanchan & Benjakul (2014), the natural essential oils are aromatic compounds present in different parts of medicinal plants, for instance: flowers, leaves, bark, roots and fruits that are separated after hydro-distillation or steam distillation as continuous solvent extraction techniques. Essential oils are a combination of low molecular mass chemical compounds, which contain: alcohols, polyphenols, terpenoids, carbonyl compounds, aliphatic substances, and they have distinct aromas and possess biological properties.
Cavalcanti (2013) and Sharmeen et al. (2021) noted that essential oils are used and applied as additives in the cosmetic, pharmaceutical, food, textile and perfumery industries to incorporate appropriate functional characteristics, ensuring a wider range of organic functions. In fact, most natural essential oils incorporate several functions, with emphasis on their use as natural dyes, nutraceuticals, functional foods, preserving agents, flavorings and fragrances, medicines, vitamin supplements, chemical standards and perfumes, among others.
Essential oils incorporate vegetable oil and are known by having an elevated content of organic and natural substances when obtained from flowers, herbs, fruits and aromatic spices. The extraction procedures involve the use of an extractor and a conventional solvent or supercritical fluid, or both, known as a hybrid mixture, where the proportion can be adjusted to improve the extraction yield (Uwineza & Waśkiewicz (2020) and Chemat et al. (2019)).
The extraction procedure used directly reflects in the quality of the products obtained, mainly in relation to the degree of purity, and the recovery capacity. The concepts of diffusion and convection mass transference, based on interfacial equilibrium between solid–liquid or solid–gas phases, are used to assess the process efficiency and to define the appropriate extraction equipment.
Boucard & Serth (2005) evaluated the extraction process based on diffusion and convectional mass-transfer phenomena to identify procedures that could enhance the quantity of the products. Shakir (2018) observed that a higher extraction temperature could increment the interaction of solvent and matrix and also the diffusion mass transfer.
Danlami et al. (2014) observed that the fundamental of solvent extraction is based on the contact of solvent and the solid material, allowing the solid materials to be transferred to the liquid phase as soluble compounds. However, in the case of plants, this extraction occurs through concentration differences. In the moment when the mass transfer decreases by the concentration of active sites, the equilibrium is finally reached and therefore the mass transfer will no longer occur.
In recent decades, an increase in international demand for essential oils produced from plants has been observed. This has driven further research to develop new extraction techniques aimed at increasing the oil quality and reducing the production cost.
In general, essential oil extraction involves vapor flow in distillation, hydro-distillation or procedures based on organic solvents, mechanical extraction or supercritical fluids (generally carbon dioxide) to improve the mass transfer during the extraction (Cavalcanti, 2013).
Filippis (2013) studied supercritical extraction with CO2 and noted the importance of the production and commercialization of essential oil globally, with a considerable variety of essential oils on the market (more than 90 types). Several important differences in the oils obtained arise from the characteristics of the process applied for the extraction.
In this context, hydro-distillation is characterized by direct contact between two phases, vapor and solid. When the vapor comes into contact with an aromatic plant, the essential oil is dragged into this phase and then recovered in a condensation system (Figure 1). In this process, to increase efficiency extraction, the processes parameters are controlled, mainly temperature, pressure, vapor flow rate and particle size of the solids. Optimizing the operational conditions in this way generally increases the extraction efficiency and yield. After this step, the essential oil produced can be purified to satisfy international standards (Golmohammadi et al., 2018).
Process adapted from Kusuma & Mahfud (2017).
When oil extraction is applied in industrial processes, the particle size is reduced and the essential oil is extracted inside an extractor tank, using appropriate solvents. After this step, the liquid phase is passed to an evaporator to remove the solvent and recover the essential oil. These processes can be applied on an industrial scale only after the associated phenomena have been studied. The difference between the ebullition temperature of essential oil and solvents ensures rapid solvent removal, using a one-stage evaporation processes. The solvent can be recovered, recycled and re-used in same extraction process, leading to a reduced need for new solvents.
For Vieira de Melo et al. (2000), the conventional procedures for essential oils recovery from plant materials, as cited above, can present limitations due to instability of essential oils by heat and by residual organic solvent from extraction. Hence, the use of SCF for essential oils extraction is being evaluated as an alternative to traditional methods.
Supercritical CO2 offers advantages because it is not toxic, not expensive, non-flammable and odor- and colorless. Also, the critical temperature is near to ambient temperature and it has low viscosity and high diffusivity in relation to liquids. Thus, it has transformed into a common solvent in the process of natural materials (Vieira de Melo et al., 2000)).
The extraction of compounds from solid matrices is associated with the internal mass transfer (dissolution and diffusion) and external mass transfer (solid particles). Thus, the solute diffused in the supercritical fluid-rich phase can be carried away by the bulk flow. Numerous authors have been published their attempts of model for these supercritical extraction process (Reverchon & Morone, 1997; Sovová, 1994; Sovová, 2005), and a broad discussion of modelling aspects is presented elsewhere (Capuzzo et al. (2013); Danlami et al. (2014); Khaw et al. (2017); Reverchon (1996); Vieira de Melo et al. (2000) and Lin et al. (2013)).
Essential oil extraction using high pressure or supercritical fluids (SCF) is an alternative from organic solvent extraction or steam distillation, and has been studied by different industries, especially in the food, pharmaceutical and cosmetic industries (Ferreira et al. (1999); Pinto et al. (1999) and Cao et al. (2007)).
Khaw et al. (2017) observed the influence of solubilization on fluid density. At a high pressure, SCF presents high density, allowing the dissolution of great quantities of organic compounds. When the density is reduced, those dissolved compounds can be recovered, and it can be performed by decreasing the pressure or increasing the temperature.
For the author cited below, by performing the separation process at lower temperatures it can avoid the degradation of compounds by decreasing the heat exposure, which occur in steam distillation.
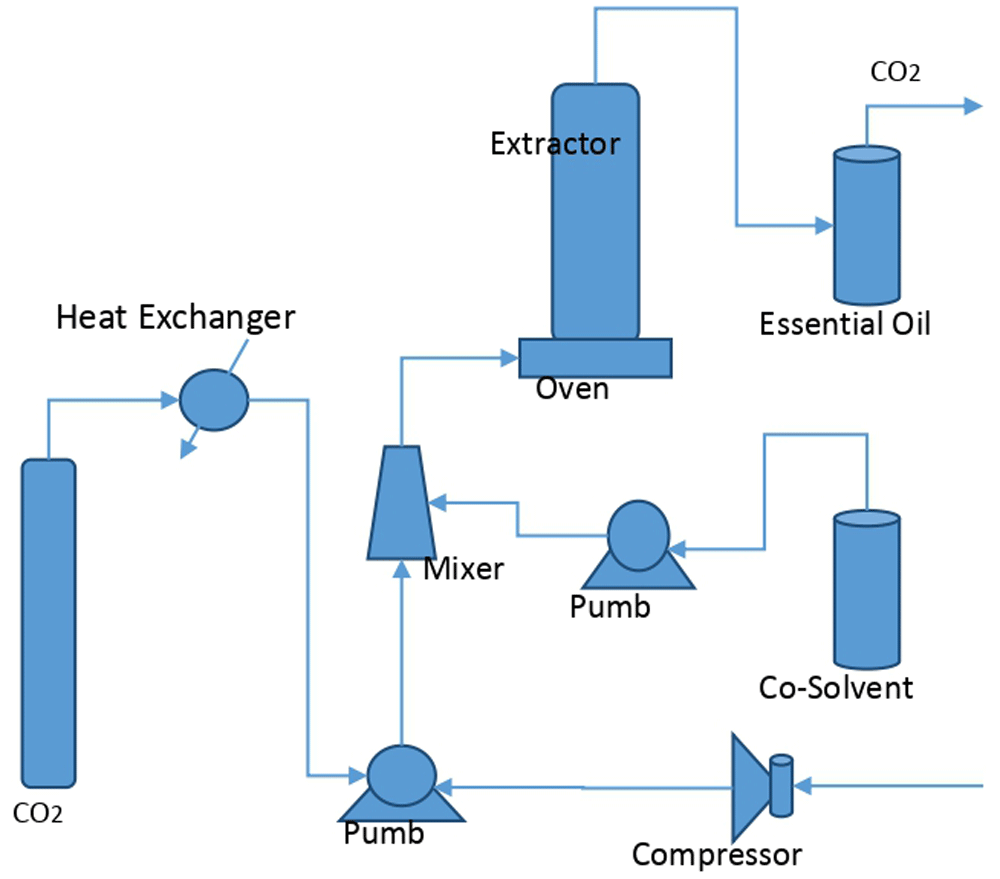
Supercritical fluid extraction provides better extraction efficiency, increases the production rate and improves the product quality. These processes, generally hybrid, involve the use of supercritical fluid, compressed air and a co-solvent, which, in combination, can intensify the fluid bubbling in the solid mass and increase the extraction efficiency (Figure 2).
Process adapted from Borges et al. (2017).
To increase the extraction efficiency with use the supercritical fluids, the extraction process must be optimized, guaranteeing the best operational conditions; mainly the temperature, pressure, flow rate, residence time, solid material size, and ratio between the material and the flow rate of the fluid used as the extractive solvent (organic solvent, supercritical fluid and compressed air).
For Manjare et al. (2019), the low polarity of supercritical CO2 is an important obstacle to its application, hindering the extraction of polar compounds. However, this could be surpassed by the addition of polar modifiers, as methanol or ethanol together with supercritical CO2, therefore increasing its solution power.
Under these conditions, it is possible to increase mass transfer rate and improve the performance of the process, guaranteeing the applicability of this type of fluid as an alternative for the improved extraction in industrial processes (Huang et al. (2016) and Silva et al. (2004)).
De Barros et al. (2014) studied supercritical extraction using laboratory and pilot scale processes. Their parametric studies supported the mathematical models developed for industrial-scale extraction, guaranteeing extraction products with high quality and greater added value. The parameters studied highlight the most appropriate and highest-performing operating conditions, allowing extraction curves to be constructed, and those arising from different procedures can be compared.
Based on these considerations, this paper proposes the extraction of essential oil, using a hybrid solvent system, involving a conventional solvent (ethanol) and a supercritical fluid (CO2). In this study, the operational parameters (temperature, pressure and solvent flow rate and mass used ratio) were controlled in order to determine the optimal condition, to increase the mass transfer and yield of the essential oil production process. The procedure used in this paper is the same used by Capuzzo et al. (2013).
In this study, for the experimental setup, an extractor system was built. The system involves the use of ethanol and supercritical fluid (CO2), as a hybrid fluid mixture, based on supercritical concepts, and the essential oil was extracted from onion. The best operational conditions were determined, aimed at market efficiency, considering the supercritical flow rate, temperature and mass of onion used in the extraction. The turbulence phenomenon was evaluated in terms of the intensity of the solid-fluid mixture, considered the velocity of the ethanol-CO2 mixture. The CO2 was bubbled in ethanol in a distillation balloon, where the mixture received the thermal energy needed to increase the solid to fluid phase transferring. The effect of onion mass on the mass transfer efficiency was also investigated.
The onion sample used in this study was previously chopped and dried in a horizontal dryer with controlled circulating air speed and the air was heated using electrical resistance, in order to improve the efficiency during the extraction process. The results are represented as graphs showing the mass loss over time.
Raw material preparation. In the study reported herein, onion (Allium cepa) was used as the raw material, which was chopped into cubes to obtain an adequate size for essential oil extraction. The chopped onion was placed in a convective dryer, coupled to an analytical balance and temperature control sensors.
The mechanical ventilation system provided airflow and there was a humidity (water concentration) gradient between the air and the onion, to increase the mass transfer from solid particles to the air.
The operation parameters for drying, hot air and mass loss of raw material were controlled. The process was carried out to give an onion mass loss of 50%, with a temperature of 40±1.2ºC, hot air speed of approximately 1.7 m/s, extraction time for 240 min and average particle size of 4 mm.
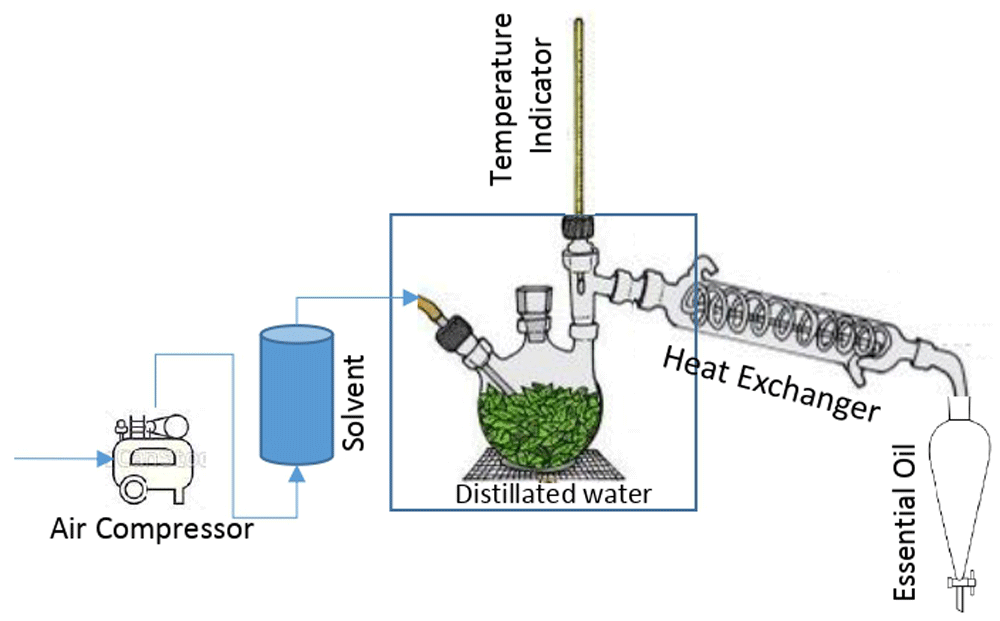
Procedure for essential oil extraction. The experimental apparatus shown in Figure 3 was used to carry out the extraction of essential oil from the dried onion.
The apparatus built for this study was comprised of three sections: a) section with fluid mixture of solvent and supercritical fluid (CO2) heated to the temperature required to provide the best conditions for the extraction process; b) the extraction of essential oil section, allowing interaction between solid and fluid phases, where the mass transfer from solid to fluid mixture occurs; c) section for recovery of essential oil, where the extraction fluid mixture carries the essential oil through the heat exchanger to the expansion tank for recover, by condensation, of the fluid mixture, ethanol and essential oil.
The experiments were performed using the apparatus (Figure 3), applying the following procedure: a) the raw material is placed inside the extraction tank and the water in the condensation system is cooled. Also, during this step, the ethanol inside the balloon is heated; b) cooled water begins to recirculate in the condenser system and when the temperature in the balloon reaches that suitable for extraction, the bottle of the CO2 valve was opened and the flow of supercritical fluid was bubbled into the ethanol to form the mixture which would pass to the extraction tank; c) the fluid mixture flows in contact with the solid phase and the mass transfer occurs. The flow then passes through the condenser to recover the ethanol and essential oil in the expansion tank.
The methodological sequence described herein was used to carry out the essential oil extraction using onion as the raw material, as well as the effect of operation parameters on the quality of the process was evaluated.
Table 1 show the operational conditions used in this study, based on an experimental plan to evaluate the parameters and their effect on the process efficiency, based on the essential oil extraction capacity.
The performance of each experiment was evaluated based on Equation 1.
The performance of each extraction essay was related to the operation parameters mainly the bubble intensity of CO2 in ethanol, temperature and fluid mixture flow rate (measured at the inlet and outlet of the expansion tank).
Essential oil purification. The essential oil produced in the extraction process describe herein was purified using simple distillation and filtration, supported by technical norms. The procedure applied provides products with a high degree of purity, suitable for application as a raw material in other industrial processes.
Characterization of essential oil. The essential oil extracted and purified was characterized to determine the acid and refraction indexes, viscosity, and specific mass. The analysis procedures are described in the standard test method ASTM D 974 and the European standard EN14103.
The results of the experimental assays described above are reported in this section along with a discussion, supported by data available in the literature, to better contribute to future scientific and technological developments.
Table 2 shows the results obtained from the drying of the raw material (onion) used in this study, based on experiments conducted using the procedure described above. The results show the mass loss over time, characterized by a loss of the water present in the structure of the raw material. The runs were conducted with control of the operation parameters.
The data in Table 2 were obtained during the drying process (240 min) and the mass was measured at 30-min intervals. Globally, for this assay, a mass loss of 892.4g corresponds to 54.42% of the initial raw material mass. These data were used to construct the graphs in Figure 4, which show the mass loss during the drying time. The drying curve indicates that a greater mass was transferred from the solid to gas phase, occurred during first hour of drying, equivalent to 39.15% of the global mass loss. In the second hour, the mass loss represents 26.45% of the global mass, in the third hour 20.80% and in the last hour 13.5%.
The decrease in water mass during drying, according to Figure 4, was associated with the mass transfer phenomenon, where the flow rate is directly related to the concentration gradients of water, between the solid and gas phases, associated with this process. In this process, mass transfer by convection at the solid surface predominates, and this is proportional to the solid area through the transfer coefficients. Equation 2 can thus describe the mass transfer, where the flow transference is related with global coefficient and the concentration gradient.
Where NA is the flow rate of water (kmol/m2s), kc is the global coefficient and ΔCA is the concentration difference between the solid particle and the airflow.
The mass loss rate was greatest during the first 180 min when approximately 65.60% of the total loss occurred. The mass transfer rates then decreased substantially leading to relative stability. The reduction in mass transfer rates is related to the drying time, which can be adjusted to guarantee the optimal condition for the essential oil extraction, as described herein.
Experimental apparatus. The experimental apparatus described above was used to carry out the procedure applied to obtain the essential oil extract from dried onion (Figure 4).
Once the apparatus had been built, we ascertained the limits operational based on experimental planning, mainly the characteristics of the mixture, supercritical fluid flow rate and the ratio of mass of conventional solvent and supercritical fluid that gives the best extraction performance.
Experimental data acquisition. Initially, the conventional procedure tested the interaction of ethanol (vapor) rate and onion (solid) mass transference from solid to vapor phase. This process, established according the solubility of the solid in the solvent, occurs through mass transfer by diffusion and convection at the solid and vapor interface. In this initial study, a Soxhlet apparatus was used to extract the essential oil from onion particles, with a global yield of the 27.93%.
Using the same conditions (Table 3), the yield increased in non-linear fashion of CO2 performance operation and reach over 60% (yield).
In all of these experiments, the operating time was the same. Aris et al. (2019) and Hassanien (2019) evaluated the effect of fluid flow rate using supercritical CO2 and concluded that when CO2 flow rate increased, the extract yield increased by 3.698% for every 0.7 mL/min. According to the cited authors, under conditions of low pressure and high temperature, a rise in the flow rate of 8 mL/min would increase extract yield. Maran & Priya (2015) and Gadkari & Balaraman (2017) observed that with an increase in the flow rate, the film thickness surrounding the solid particle reduced, thus decreasing the external mass transfer resistance around the solid. The solute could thus easily move to the bulk solvent, enhancing the solubilization of the solute in the solvent and increasing the extract yield.
Özkal & Yener (2016) observed that when the flow rate of the supercritical fluid was increased considerably, there was an increase in the convection between solid and CO2. In this case, this causes damage to the weak parts of the solid particles, leading to freer solute being removed from the solid particle during extraction.
Table 3 shows the yield effect by the CO2 flow rate in terms of essential oil recovery, related to the role of the solubility of the oil on the concentration in the supercritical fluid flow.
In a study by Inamuddin & Asiri (2020) and Da Silva et al. (2016), the extraction yield with ethanol, at high CO2 solvent pressures, increased with a decrease in pressure in the binary CO2-ethanol system, and the extraction yield was high.
In the study reported herein, a hybrid system was used, with supercritical fluid and ethanol as the extraction fluid. In this case, the bubbling of the supercritical fluid in a conventional solvent was used to form the mixture, which was applied in the extraction of essential oil. Interaction between the solid and vapor phases was verified.
In all cases evaluated, the essential oil was characterized in terms of the acid index and refraction index (Table 3). The values obtained for the three runs were 1.132; 1.130 and 1.127 g/mL, which are within limits reported in literature. The average value obtained for the acid index in these the experiments was 3.19 mg KOH/g, while data obtained from the literature show an average value 3.56.
We can conclude:
a) The essential oil from supercritical fluid and conventional solvent mixture extraction provided good yields;
b) The use of CO2 in the extraction processes is a good alternative to improve the extraction performance, due to the turbulence imposed on the mixture in the extractor, which increases the mass transfer between solid and fluid phases;
c) The mass transfer by convection in essential oil extraction, based on the results of this study, is more effective than that by diffusion, mainly with the use of a supercritical fluid in the system; and
d) The apparatus proposed for this study represents a good alternative to improve the extraction of essential oil, with a view to obtaining new products for use as raw materials in other industrial processes.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Emerging/unconventional extraction techniques.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Extraction, food technology, analytical chemistry
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Extraction of vegetable oil, extraction of essential oil, supercritical fluid extraction
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
References
1. Ye C, Lai Y: Supercritical CO2 Extraction Optimization of Onion Oil Using Response Surface Methodology. Chemical Engineering & Technology. 2012; 35 (4): 646-652 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Supercritical fluid extraction, Biodiesel production, Subcritical water extraction, Lipid, Essential oil
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Process separation; green chemistry; chemical engineering.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biotechnology, Screnning of enzymes, Enzyme purifications; Reconbinant process on biotecnhology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
|
Version 1 21 Jul 21 |
read | read | read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)