Keywords
Critical period, IEG, Somatosensory cortex, Ccular dominance, Cortical development, Plasticity
Critical period, IEG, Somatosensory cortex, Ccular dominance, Cortical development, Plasticity
Postnatal development of the neocortex includes crucial stages when proper experience defines the subsequent maturation of sensory, motor, cognitive and other functions. There is a defined time window, described as a critical period (CP), when a particular developing brain structure is especially sensitive to environmental stimuli and when a relevant experience can produce large-scale permanent changes to neural circuits1–7. CPs are characterized by exceptionally high levels of plasticity, and have been best documented for sensory systems, particularly for the development of vision1,4,7–9. Experience-dependent modification of the mammalian visual cortex (VC) occurs during a narrow temporal window and is indispensable for the subsequent proper connectivity and vision development. Perturbations during the CP of the visual system development lead to deficiencies in the processing of the visual signals, and in humans may result in disorders of sight such as amblyopia4,10,11.
The development of the primary visual cortex (V1) in mice occurs in several stages7. The first stage includes formation of precise topographic maps; it takes place before the eyes opening and before the axonal projections from the dorsal lateral geniculate nucleus organize high-resolution point-to-point connections with neurons in layer 4 of V1 (L4). During the second stage of V1 development, around the time of eye opening and within several days of the first visual responses in the retina, V1 neurons acquire orientation selectivity. In the third stage of V1 development, the selective properties of neurons are refined and become similar through both eyes. For the mouse VC, the CP corresponds to PND21–35. After that period, the circuitry and responses of V1 appear mature and normally remain stable throughout life7.
Although a large part of the VC corresponds to a monocular zone that receives inputs predominantly from the contralateral eye, a smaller binocular zone of V1 receives inputs from both eyes. Neuronal responses in the binocular region are driven more strongly by visual inputs received from the contralateral eye than the ipsilateral eye, in a phenomenon characterized as ocular dominance (OD). OD is highly influenced by visual experience during CP and changes in OD (OD plasticity) are possible only during early postnatal development through monocular deprivation of visual stimuli3,4,7.
CPs in brain development are associated with pronounced changes in gene expression, driven by the changes in expression of key transcriptional regulatory factors5,12. For instance, the highest levels of pCREB, the phosphorylated (active) form of the transcriptional factor CREB, are found during and after the CP of VC development13,14. Activation of CREB induces expression of its downstream targets, such as immediate early genes (IEGs) c-Fos, Egr1, or Arc, which are themselves important regulators of gene expression cascades; moreover, induction of IEG expression (e.g., of c-Fos) is often considered a proxy of neuronal activation. Changes in IEG expression have been thoroughly investigated for the developing visual cortex of mice, rats, cats, and primates8,15–35, with the results providing strong evidence for the regulatory input of IEGs into the development of the visual system. Such studies have been particularly informative for the mouse model, with the conclusions being extrapolated to human brain development and human vision disorders3,4,10,11. Here we asked whether c-Fos and Egr1, two IEGs broadly used as markers of neuronal activity, show distinct patterns of activation in the mouse brain, as related to the CP of the visual system development. Towards this goal, we explored the dynamics of basal and induced expression of c-Fos and Egr1 in the binocular zone of V1 at different stages of mouse VC development – before, during, and after the CP, when they may be required for the proper development of vision.
In this study we used C57Bl/6J mice of both sexes (RRID:IMSR_JAX:000664, The Jackson Laboratory, USA). All experiments were performed in accordance with the requirements of the Ministry of Health of the Russian Federation (Decree no.267 of 19/6/2003) and the “Regulations for studies using experimental animals” (Research Institute of Normal Physiology, Russian Academy of Medical Sciences, Protocol no.1 of 3/9/2005).
Before the experiment, animals were kept under normal conditions at room temperature in 12/12h light-dark cycle and maintained on ad libitum food and water. For breeding, adult animals (one male and 2–3 females) were kept together in the colony room in standard cages 36×21×13.5 cm. Pregnant dams were transferred to individual cages a few days before labor, and appearance of pups was taken for the first day of postnatal development (PND1). Litters of 6–8 pups were considered normal and selected for the experiments. Pups from each litter were kept with mothers until PND21. In further experiments each pup was considered to be an experimental unit.
Until the end of the experiment, the animals were not subjected to pain or stressors. Pups were kept with dams (without other mice in the cage) until the end of weaning period to prevent infanticide and to ensure comfortable conditions for the animals. The deprivation camera was well ventilated and quiet. All manipulations were performed with the utmost speed and care. PND16 mice were euthanized by transposition of cervical vertebrae to minimize animal distress as recommended for the juvenile mice by the American Veterinary Medical Association (AVMA) Guidelines for the euthanasia and the European Commission. We applied the same method to the mice of other ages for experimental consistency.
A total of 51 mice were divided into three groups: home cage group (HOME), a total of 15 animals: n=4 for PND16, n=5 for PND30 and n=6 for PND55; dark control group (DARK), a total of 17 animals: n=6 for PND16, n=6 for PND30 and n=5 for PND55; and experimental group (EXP), a total of 19 animals: n=5 for PND16, n=7 for PND30 and n=7 for PND55. The number of animals per experimental group was based on the information on the variation of the response as measured by the number of c-Fos-expressing cells and mean and standard deviation of the response, as obtained from our previous experience and pilot studies. This information was used for statistical power analysis to estimate the group size that is necessary to test our hypotheses, with significance level alpha of 0.05 (95%).
The average weight of an animal of the corresponding age was 4–8g for PND16, 10–20g for PND30 and 20–25g for PND55. Atypically low weight of the animal at the time of euthanasia was considered as an a priori exclusion criterion. All animals in this series met this inclusion criterion and were therefore used for the experiments.
On PND9, PND23, or PND48 (i.e., before, during and after CP), the cages containing mice from the EXP and DARK groups were placed into a light deprivation camera (a ventilated plastic box, 55×39×46.5сm, fully isolated from external light) and kept there for 2 days in a standard light-dark cycle, with two cages in the camera at a time. For the next 5 days, the EXP and DARK groups were deprived of light. Deprived mice from each cage were randomly allocated into DARK or EXP group by the experimenter so that each group was sex-balanced. On PND16, 30 or 55, respectively, EXP mice were exposed to bright light for 90 min before euthanasia; DARK mice were euthanized immediately after opening of the deprivation camera. HOME mice were maintained under a standard light-dark cycle for the entire time before euthanasia.
After euthanasia, brains were quickly removed and frozen under liquid nitrogen vapor. Investigators could not be blinded before collecting brain samples due to different location of the HOME group and the time of euthanasia of the DARK and EXP groups. Collected brain samples were double-blind coded and decoded only after all images were obtained and cell counting was completed.
Serial 20 µm thick coronal sections through the VC and SC were cut with a freezing microtome (Leica CM 1950, Germany). Sections were fixed in 4%PFA for 10 min at 4°C, rinsed in 1x PBS three times for 5 min each at RT and placed in an immunostaining chamber (Sequenza Immunostaining Center, Shandon, UK). Sections were incubated with 2.5% normal horse serum (Vector Laboratories, USA) in 1x PBS for 40 min at 4°C and then with primary polyclonal antibodies against c-Fos (anti-rabbit, 1:500, sc-52, RRID:AB_2106783, Santa Cruz Biotechnology, USA) or Egr1 (anti-rabbit, 1:1000, sc-189, RRID:AB_2231020, Santa Cruz Biotechnology, USA) in 1x PBS, 2.5% normal horse serum and 0.01% NaN3 overnight at 4°C. After 3 rinses in 1x PBS, the sections were incubated with secondary biotinylated anti-rabbit IgG polyclonal antibody (ImmPress PK-7401, RRID:AB_2336529, Vector Laboratories, USA) for 50 min at RT. After being rinsed three times in 1x PBS, the sections were incubated in DAB solution (0.06% DAB and 0.003%H2O2 in 1x PBS) for 5–7 min at RT, rinsed again with dH2O, dehydrated with a graded concentrations of ethanol series (70%EtOH, 96%EtOH, and 100%EtOH) and xylene for 5 min each step and then coverslipped with Mount-Quick (DAIDO SANGYO Co, Japan).
The brain sections (three coronal sections per brain) were captured with a computer assisted microscope system Olympus VS110 (10x, NA 0.40, Olympus, Japan). Images were converted into gray scale in Photoshop CS5 v.12.0.1 (RRID:SCR_014199, Adobe Systems Inc., USA), and the number of c-Fos+ or of Egr1+ cells was counted in the VC and SC with ImagePro Plus v.3.0 software (RRID:SCR_016879, Media Cybernetics, USA). Cell density in the region structure (cell number/area of the structure, mm2) was calculated and statistically analyzed with a set of density values for different sections of the same region averaged over the number of slices.
At this stage, a priori criteria for excluding animals from the analysis were the absence (mainly due to cutting or staining errors) of three slices from the selected region (mainly due to cutting or staining errors); immunohistochemical staining of improper quality (too pale or too bright); or poor morphology of slices that did not allow counting the number of cells in all three slices from the region. As a result of applying these criteria, 1–2 animals from some Egr1 groups were excluded. The final number of 51 mice remain unchanged for the statistical analysis of c-Fos. Egr1 expression was analyzed in total of 43 animals: a total of 15 mice in the EXP group: n=5 for PND16, n=5 for PND30, and n=5 for PND55; a total of 15 mice in the DARK group: n=5 for PND16, n=5 for PND30, and n=5 for PND55; and a total of 13 mice in the HOME group: n=4 for PND16, n=4 for PND30 and n=5 for PND55.
Statistical analysis was performed in Statistica v.7 (RRID:SCR_014213, StatSoft) and GraphPad Prism 6.01 (RRID:SCR_002798, GraphPad Software). Kruskal-Wallis test with multiple comparisons of mean ranks for all groups (KWmc test) used for intergroup comparisons. For the comparison of VC and SC, Wilcoxon matched pairs test (Wmp test) was used. Differences were considered statistically significant at p < 0.05. All data are presented as a mean ± standard error of the mean.
To investigate the CP-related dynamics of the cortical cell populations carrying markers of neuronal activation, we first defined the basal level of c-Fos-expression in the brains of the home caged mice (control HOME group mice). The brains were analyzed for c-Fos-expressing cells in the primary VC before (PND16), during (PND30), and after (PND55) the CP. In parallel, we determined the changes in c-Fos+ cells in the barrel field of the primary SC of the same brain preparations. We found very few c-Fos expressing cells (1–10 cells per mm2) in both the VC and SC at each of the three analyzed time points: 0.84±0.42 in the VC and 0.39±0.15 in the SC at PND16; 1.45±0.45 in the VC and 1.27±0.14 in the SC at PND30; and 10.17±4.96 in the VC and 2.83±1.14 in the SC at PND55 (Figure 1A and B).
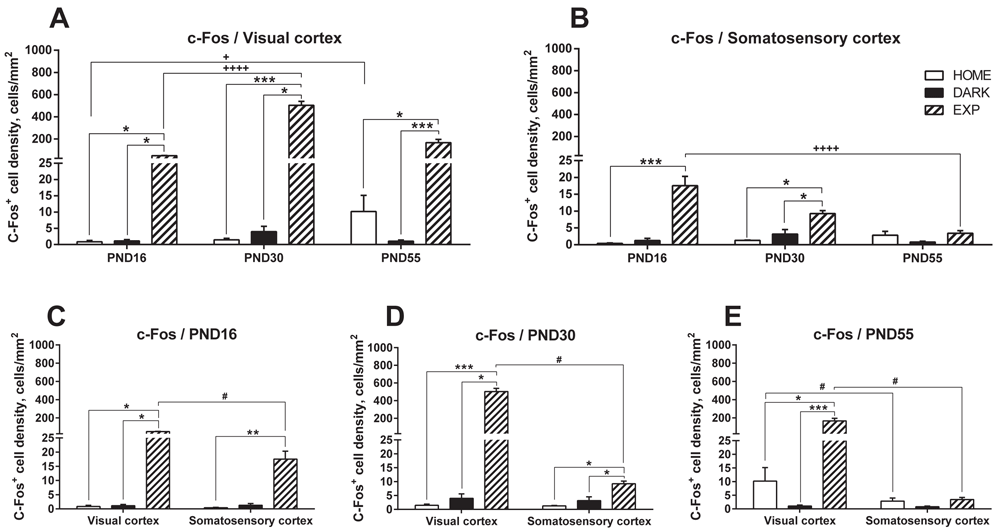
For clarity and comparable axes values, the data are presented as grouped by the cortical areas: VC (A) and SC (B) and as grouped by days: PND16 (C), PND30 (D), and PND55 (E). Data for the HOME, DARK and EXP groups at the same age are compared using KWmc test for all groups, with * - p<0.05; ** - p<0.01; *** - p<0.005. Data for the same group at different ages are compared using KWmc test, +++ - p<0.005. Data for VC and SC are compared using Wmp test, # - p<0.05.
Next, we determined c-Fos expression in mice deprived of light for five consecutive days (the DARK group). In these dark-reared animals, similarly to the HOME group, the number of c-Fos-expressing cells was very low in both the VC and SC at each of the three time points: 1.12±0.44 in the VC and 1.26±0.62 in the SC at PND16; 3.96±1.64 in the VC and 3.17±1.34 in the SC at PND30; and 1.05±0.35 in the VC and 0.80±0.24 in the SC at PND55 (Figure 1A and B).
We then determined whether brief exposure to light (the EXP group) altered c-Fos expression. Indeed, at each analyzed time point the number of c-Fos-positive cells was significantly higher in both the VC and SC in the EXP group than in the HOME or DARK groups. On PND16, before the visual input CP, c-Fos expression in EXP mice was 49.32±2.36 in the VC and 17.53±2.78 in the SC (Figure 1A and B), i.e., 47- and 35-fold higher (VC) and 36- and 11-fold higher (SC) than that in the HOME and DARK groups, respectively (p=0.0048, KWmc test). Note that c-Fos induction was 2.8-fold higher in the VC than in the SC at this time point (p=0.04, Wmp test, Figure 1C).
During the CP, at PND30, c-Fos expression in EXP mice was also significantly higher than that in the HOME and DARK groups in both cortical areas: 502.98±35.76 cells in the VC and 9.24±0.91 cells in the SC (Figure 1A and B). For the VC this corresponded to a level 350- and 127-fold greater than those in the HOME and the DARK group, respectively and for the SC this corresponded to levels 7.7- and 2.9-fold greater than those in the HOME and the DARK groups (respectively, p=0.004 and 0.018 for VC and p=0.016 and 0.035 for SC in KWmc test). As it was observed for PND16, induction of c-Fos after light exposure was significantly higher in the VC than in the SC (54-fold, p=0.018, Wmp test, Figure 1D).
Finally, after the CP, on PND55, exposure to light also resulted in a significant increase in c-Fos expression in the VC (166.73±29.59 cells, Figure 1A and E), which corresponded to a level 16.4- and 154-fold greater than that in the HOME and DARK groups (p=0.038 and 0.002, respectively, KWmc test for all groups). In the SC, in contrast to the PND16 and PND30 results, light exposure did not increase the number of c-Fos-expressing cells (3.41±0.76, Figure 1B and E) and there was no statistically significant difference relative to the HOME and DARK groups.
In parallel to the analysis of c-Fos-expressing cells, we analyzed the number of Egr1-expressing cells in the same preparations. On PND16, the number of Egr1-expressing cells was comparable in all groups in both cortical areas (Figure 2A-C): 632.23±49.70, 605.93±100.22, and 599.24±45.65 cells/mm2 in the VC in the HOME, DARK, and EXP groups, respectively, and 564.93±53.47, 532.04±88.36, and 720.70±72.60 cells/mm2 in the SC in the HOME, DARK, and EXP groups.
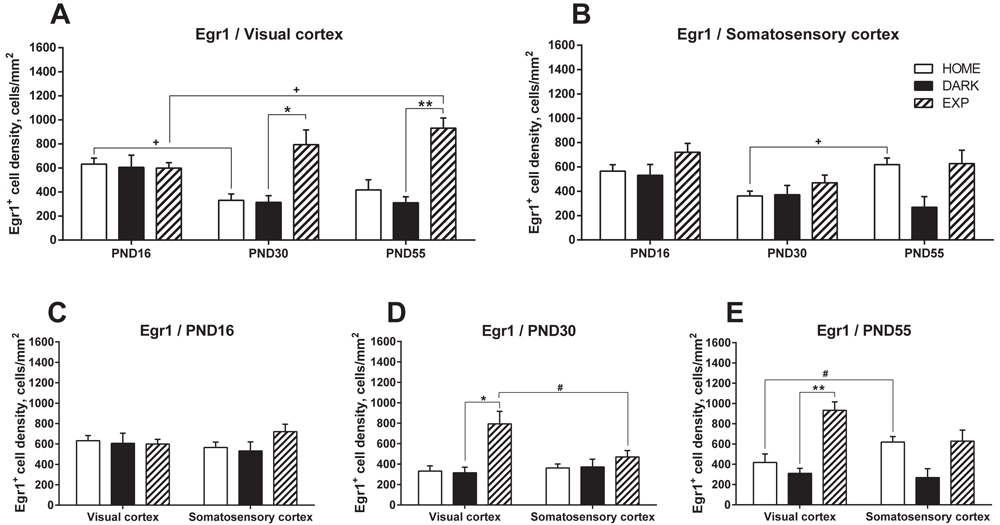
For clarity and comparable axes values, the data are presented as grouped by the cortical areas: VC (A) and SC (B) and as grouped by days: PND16 (C), PND30 (D), and PND55 (E). Data for the HOME, DARK and EXP groups at the same age are compared using KWmc test for all groups, with * - p<0.05; ** - p<0.01; *** - p<0.005. Data for the same group at different ages are compared using KWmc test, +++ - p<0.005. Data for VC and SC are compared using Wmp test, # - p<0.05.
On PND30 (Figure 2A, B and D), i.e., during the CP, the basal level of Egr1 expression (HOME group) in the VC was significantly lower than that on PND16 (331.36±52.20, p=0.04, KWmc test). However, light exposure produced significant induction, with 793.85±122.71 Egr1-expressing cells/mm2, a level 2.5-fold greater than that in the DARK group (p=0.038, KWmc test). This induction was specific to the VC and was not observed in the SC at that time point. In the VC, as compared with the SC, the number of Egr1-cells in the EXP group was 1.7-fold higher (p=0.043, Wmp test).
On PND55 (Figure 2A, B and E), light exposure produced a significant induction of Egr1-expressing cells in the VC (3.3-fold greater levels in the EXP group (931.67±84.13 cells/mm2) than in the corresponding DARK group (310.51±49.89 cells/mm2), p=0.007, KWmc test), but not in the VC or SC in the other groups. When compared across the time-points, the number of Egr1-expressing cells increased at PND55 in the VC (1.55-fold increase over the PND16 values) but not the SC.
Several attempts have been made to identify genes differentially expressed in the VC before and after the CP, and c-Fos and Egr1 have been suggested to act as important modulators of the CP4,5,8,9,36,37. Our results support this possibility by showing time- and region-specific response of the VC to visual stimuli, as revealed by the induction of c-Fos-positive cells and, to a lesser degree, of Egr1-positive cells.
We investigated the dynamics and specificity of the IEG induction at the key stages of the mouse cortical development – before, during, and after the visual CP. Our results show that exposure to light at the CP (PND30) results in a markedly high (130–350-fold) increase in c-Fos-expressing cells in the VC relative to the levels in the DARK and HOME groups, and to the levels before and after the onset of CP, and relative to an adjacent unrelated cortical area (SC).
c-Fos induction in response to light was also observed before the CP, but to a lesser degree (11–36-fold). Strong increase in c-Fos+ cells in the VC was also observed after the CP (153-fold at PND55). Induction of c-Fos-expressing cells in response to light was also observed in the SC during the CP, albeit at a significantly smaller scale. This increase in the SC disappeared after the CP, by PND55.
Egr1, another potential marker and effector of CP, responds to light during the CP by increased induction in the VC, although at a much smaller scale than c-Fos (a 2.5-fold increase). Induction of Egr1-positive cells was also preserved after the CP. The overall number of Egr1-expressing cells in the VC in response to light was similar before, during, and after the CP (with a small 1.55-fold increase from PND16 to PND55). Thus, although Egr1 responds to light during the CP, its specificity with respect to the CP appears to be less than that of c-Fos. At the same time, a lack of Egr1 response before the CP may reflect a shift in the role of Egr1 during development and its involvement in the plastic changes occurring during the CP, such as changes in the excitatory/inhibitory balance.
The validity of c-Fos and Egr1 as markers of the visual response, was corroborated by a much less pronounced induction of c-Fos (and no significant induction of Egr1) in the somatosensory, as compared to the visual, cortex. At the same time, the SC still responded to light with a robust increase in c-Fos+ cells, which was particularly pronounced before the CP, when the c-Fos induction was close to that in the VC (35–47-fold in the VC vs. 11–35-fold in the SC). This cross-modal activation was transient: it was less pronounced during the CP and disappeared after the CP. Cross-modal activation was not observed for Egr1, which may indicate greater specificity of Egr1 in the plastic rearrangements accompanying cortical maturation.
In sum, our results demonstrate dynamic changes in the expression of IEGs and suggest that IEG activation cascades underlie high cortical plasticity observed during the critical periods of visual cortex development.
Dryad: Differential activation of c-Fos and Egr1 during development of the mouse visual cortex. https://doi.org/10.5061/dryad.k0p2ngf7938.
This project contains the following underlying data:
- Data file 1: Egr 1 cell density in PND 16 mice
- Data file 2: Egr 1 cell density in PND 30 mice
- Data file 3: Egr 1 cell density in PND 55 mice
- Data file 4: Fos cell density in PND 16 mice
- Data file 5: Fos cell density in PND 30 mice
- Data file 6: Fos cell density in PND 55 mice
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We thank Drs. Irina Zaraiskaya (P.K. Anokhin Institute of Normal Physiology) and Evgeny Amelchenko (Stony Brook University) for critical reading of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience, immmunohistochemistry, animal models
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroplasticity and neocortical circuits
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 08 Feb 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)