Keywords
COVID-19, efficacy, safety, mesenchymal stem cell.
COVID-19, efficacy, safety, mesenchymal stem cell.
Coronavirus disease 2019 (COVID-19) is a new disease that resembles pneumonia. As of August 2021, more than 200 million people worldwide have been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus that causes COVID-19, and more than four million people have died.1 In Indonesia alone, COVID-19 has reached almost four million cases (as of August 2021) with more than 120,000 deaths.2
Besides fever, cough, and shortness of breath that are typical of pneumonia, COVID-19 has various clinical manifestations ranging from sore throat, fatigue, dizziness, myalgia, chest pain, rhinitis, neurological manifestations, conjunctivitis, anorexia, diarrhea, skin manifestations such as rashes and urticaria, and some are even asymptomatic.3–5 Therefore, COVID-19 is also known as “the great imitator”. It is interesting that COVID-19 is less severe in children than in adults, while the elderly and people with comorbidities such as hypertension, obesity, diabetes mellitus, cardiovascular disease, malignancies, and other chronic diseases, tend to have more severe symptoms and have a higher mortality rate.3,6,7
Even though it has a mortality rate that is not too high (case fatality rate of 2-3%), deaths from COVID-19 should not be underestimated.3 Various types of treatment for COVID-19 have been observed in trials such as various antivirals, dexamethasone, anti-inflammatory drugs, and even convalescent plasma therapy. However, most of these treatments have not proved effective and only the drug, remdesivir, has been shown to be effective and approved by the FDA (US Food and Drug Administration). Therefore, currently the majority of COVID-19 patients are treated symptomatically.8 Although the majority of patients have mild symptoms and recover without the use of mechanical ventilation, severe respiratory distress may occur in some patients, which can result in mortality.9 Therefore, various studies are still being conducted to find other potential treatments in COVID-19 management, one of which is stem cell therapy.
Mesenchymal stem cells (MSCs) are a type of multipotent stem cells in adults that can be found in various autologous and allogeneic sources, which have high proliferative abilities and can differentiate into various lineages. Several studies have shown that MSCs have immunomodulatory abilities that might help modulate proliferation, activation, and function of a wide variety of immune cells, both innate and adaptive cells. MSCs were previously used in graft vs host disease management and several immunological diseases caused by viruses such as HIV, chronic hepatitis B, and acute lung injury caused by influenza virus.9
Excessive inflammatory reaction is found in COVID-19 patients due to the production of inflammatory factors including cytokines, chemokines, and reactive immune cells that cause cytokine storms. MSCs administration is thought to reduce immune reactions in COVID-19 patients, therefore preventing cytokine storms from occurring in the immune system and triggering endogenous repair. After intravenous injection is given, the trapped population of mesenchymal stem cells in the lungs functions to reduce inflammation by releasing anti-inflammatory mediators, improve lung microenvironment by releasing antimicrobial peptides, protect alveolar epithelial cells, prevent pulmonary fibrosis, and improve pulmonary dysfunction and pneumonia due to COVID-19.10,11
There are many advantages that MSCs have compared to other therapies, 1) mesenchymal stem cells can be extracted from various tissues such as bone marrow, adipose tissue, umbilical cord, dental pulp, menstrual blood, buccal fat tissue etc; 2) these stem cells are multipotent; 3) mesenchymal stem cells can be stored for repetitive use; and 4) there have been no studies showing adverse events of allogeneic stem cells.10
Many studies have been conducted globally on the efficacy of MSCs against COVID-19. A case report from China by Liang et al.,12 reported a 65-year-old woman infected with SARS-CoV-2 who showed improvement in vital signs, blood and immune profiles, and CT scan results after MSC administration from umbilical cord. A randomized controlled trial conducted in the United States by Lanzoni et al.,13 reported that COVID-19 patients who were given stem cell therapy experienced significant symptom improvement compared to the control group and no adverse event was reported. However, one study reported a high incidence of adverse events.14 These controversial findings prompted the authors to conduct a systematic review and meta-analysis of MSC safety and efficacy in COVID-19 patients.
We included interventional studies with/without randomization and blinding. Each study must’ve reported evidence of ethical clearance by the local research ethics committee. Only articles in English with a full manuscript available were included. The type of intervention was MSC administration, without any restrictions on cell seeding/harvesting method and MSC dose. There were no restrictions on the patient's social status, ethnicity, race, or nationality. The expected comparisons were administration of a standard therapy regimen according to local health protocols, placebo, or MSC vehicle.
We excluded studies that included patients with: 1) age under 18 years (pediatric patients); 2) multiorgan failure at the start of study; or 3) inherited/acquired immunity disorder. Each study reported at least one of these variables: mortality rates, adverse events, need for ventilators, treatment duration, time for clinical improvement, and changes in inflammatory markers. Case reports or case series studies were also excluded from this systematic review. Mortality was defined as deaths that occurred during hospitalization. Adverse events were evaluated within six hours of MSC administration, which included urticaria, palpitations, and pulmonary edema. Cardiac arrest within 24 hours after MSC administration was also considered as an adverse event.
A systematic literature search of (Pubmed/MEDLINE, EBSCO/CINAHL, ProQuest, and Scopus from 1 January 2020 to 20 February 2021 was performed using the search strategy outlined in Table 1.15 Additional records were retrieved from Google Scholar. Duplicate articles were removed after the initial search. Two authors independently screened the title and abstract of the articles. Articles that passed the screening were assessed in full text based on the eligibility criteria. Disagreements were resolved by discussion with the senior author. This study was conducted following the Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA) statement.16
We assessed the suitability of each study that appeared in the search engines by title and abstract. All appropriate articles were input in our database and duplicated using Zotero 5.0 (RRID:SCR_013784) citation manager application. All authors conducted an independent assessment of the eligibility of all studies. At this stage, the article eligibility assessment was conducted based on the full text of the assessed article. Any discrepancies that occurred were resolved by discussion.
All authors extracted the data independently. We developed a data extraction sheet that was referred to by the Cochrane Consumers and Communication Review Group.15,17 Some of the data we extracted included study design, age, number of participants, comorbidities, type of intervention, and all of the previously mentioned outcomes. Numerical representations were preferred over graphs to avoid misinterpretation/estimation. However, graphic presentation in each article was not a reason to exclude an article.
This meta-analysis compared MSC safety and efficacy in COVID-19 patients against primary (mortality) and secondary (adverse events, treatment duration, need for mechanical ventilation) clinical outcomes. For treatment duration, the input data are numerical, therefore we used the mean difference from each study to be processed in this meta-analysis. We used a 2 × 2 format to assess mortality, side effects, and need for mechanical ventilation outcomes, therefore OR (Odd Ratio) were obtained from each study. We compiled all the outcomes from each study by considering their weight, using the RevMan 5.0 (RRID:SCR_003581) application. Studies with high heterogeneity were analyzed using the DerSimonian and Laird random-effects model and Mantel-Haenszel fixed effect model was used if heterogeneity was low.
Quality assessment was conducted by two independent reviewers (A and B). If there were differences in bias assessment results, a senior reviewer (C) was involved in making the final decision. Bias risk assessment in this study used the Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool and the revised Cochrane risk-of-bias (RoB) tool for randomized trials.18 The RoB instrument was used to assess the quality of interventional randomized studies with/without blinding.18 The ROBINS-I instrument was used to assess bias risk in interventional studies without randomization, according to Cochrane recommendations. The ROBINS-I tool used low, moderate, and serious as terminologies for risk stratification of the assessed studies.18
There were 137 articles that appeared in the initial search of the entire electronic database. Checking for duplicated articles was conducted using Zotero 5.0 (RRID:SCR_013784) citation manager application, where 133 articles were found free from duplication. We screened articles based on title and abstract suitability, and only seven articles matched. Two articles were excluded because they did not use controls (comparators) and did not report outcomes that matched the outcomes set in this meta-analysis. The PRISMA flow diagram detailing the literature search can be seen in Figure 1.
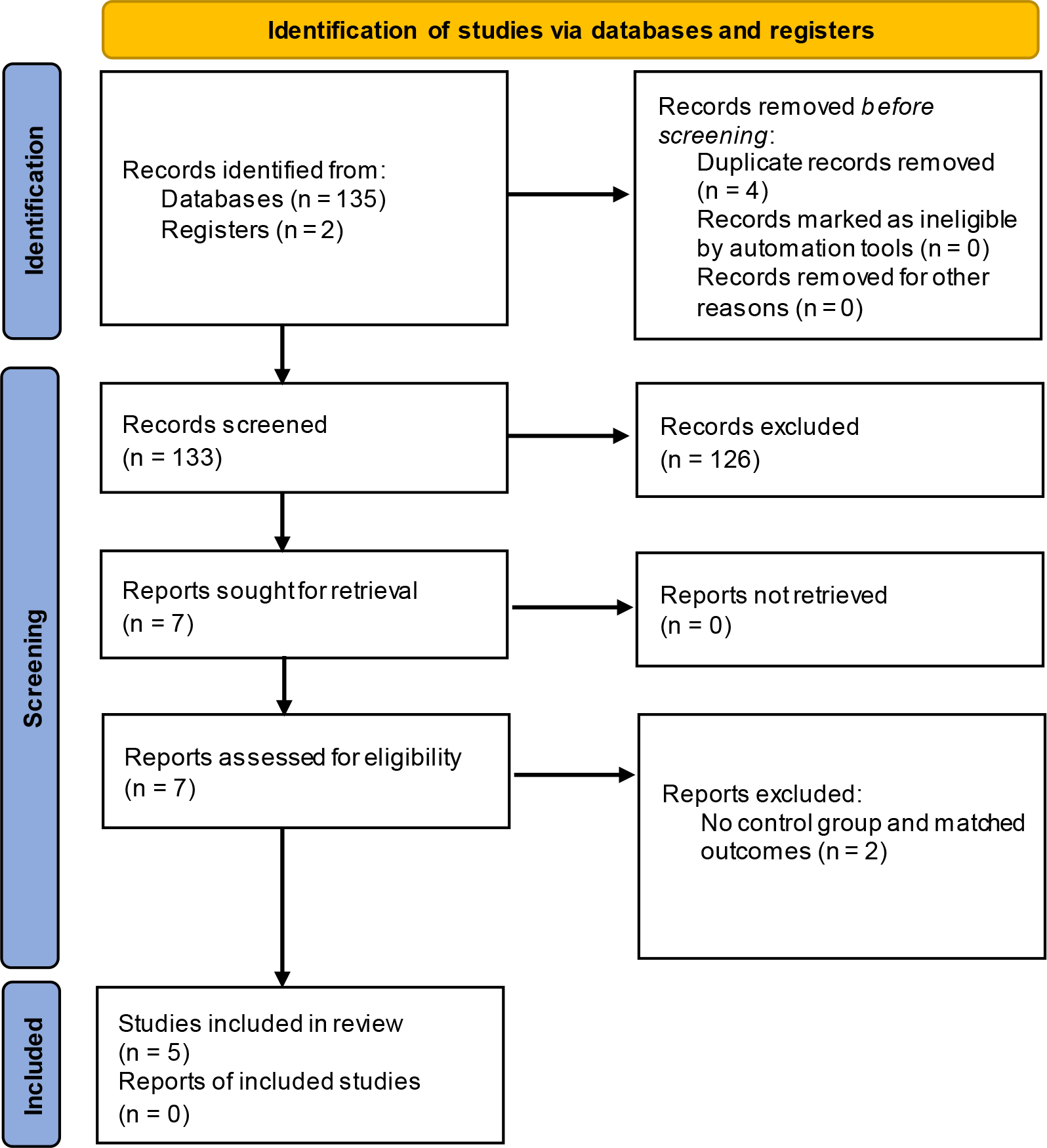
Five studies were included from 137 results of the literature search.
We included five interventional studies with a total of 193 participants. Two out of the five studies did not apply randomization. One of the three randomized studies did not apply blinding to either participants or medical professionals. MSCs were taken from human umbilical cord, except for Leng et al., who did not specify the MSC origin used.19 Shi et al., administered MSC therapy at a dose of 2 × 106 cells/kg,20 while Lanzoni et al., used a fixed dose of 100 ± 20 × 106 cells.21 The other three studies did not mention the MSC dose administered explicitly.
All studies excluded pediatric patients. The mean age of participants across the studies was over 45 years. Meng et al., and Leng et al., excluded patients with comorbidities.14,19 The other three studies included comorbid patients, with a distribution/proportion that did not differ significantly between groups. The study characteristics and outcomes reported for each study can be seen in Tables 2 and 3.
| Authors | Design | N | Age | Comorbidities | Intervention | Outcomes |
|---|---|---|---|---|---|---|
| Lanzoni et al., 2021.20 | Randomized and double-blind interventional study | 12 (I) 12 (C) | 58.58 ± 15.93 (I) 58.83 ± 11.61 (K) | Diabetes (45.83%); hypertension (66.67%) | IV infusion of 100 ± 20 × 106 umbilical cord-derived mesenchymal stem cells | Adverse events, mortality, inflammation markers |
| Leng et al., 2020.18 | Interventional study without randomization | 7 (I) 3 (C) | 57 ± 7.75 (I) 65 ± 16.46 (K) | NR | Mesenchymal stem cells infusions* | Adverse events, mortality, inflammation markers |
| Meng et al., 2020.14 | Interventional study without randomization | 9 (I) 9 (C) | 45.11 ± 10.13 (I) 49.55 ± 10.06 (K) | NR | Umbilical cord-derived mesenchymal stem cells infusions* | Adverse events, treatment duration, need for ventilator inflammation markers |
| Shi et al., 2020.19 | Randomized and double-blind interventional study | 65 (I) 35 (C) | 60.72 ± 9.14 (I) 59.94 ± 7.79 (K) | Diabetes (17%); hypertension (27%)** | Umbilical cord-derived mesenchymal stem cells infusions* | Adverse events, mortality, need for ventilator |
| Shu et al., 2020.21 | Randomized and non-blinded interventional study (open label) | 12 (I) 29 (C) | 61.00 ± 17.87 (I) 57.86 ± 15.79 (K) | Diabetes (19.51%); hypertension (21.95%)** | 2 × 106 Umbilical cord-derived mesenchymal stem cells/kg | Adverse events, mortality, treatment duration, need for ventilator inflammation markers |
| Authors | Adverse events | Mortality | Need for ventilator | Treatment duration | Study quality |
|---|---|---|---|---|---|
| Lanzoni et al., 2021.20 | 0.18 [0.02, 1.95] | 0.07 [0.01, 0.75] | Not reported | Not reported | Good |
| Leng et al., 2020.18 | None* | 0.11 [0.00, 3.70] | Not reported | Not reported | Moderate |
| Meng et al., 2020.14 | 10.23 [0.45, 233.23] | Not reported | 0.16 [0.01, 1.83] | −2.75 [−4.62, −0.88] | Good |
| Shi et al., 2020.19 | 0.88 [0.38, 2.03] | None* | 2.80 [0.13, 59.86] | Not reported | Good |
| Shu et al., 2020.21 | None* | 0.30 [0.01, 6.32] | 0.29 [0.03, 2.62] | −4.00 [−5.44, −2.56] | Moderate |
Study quality assessment based on risk of bias using the RoB and ROBINS-I tools showed that the included studies were of moderate to good quality (Figure 2 and Table 4). A study by Leng et al., had moderate quality because there was no difference in the analysis of group characteristics.19 This might lead to confounding bias. Furthermore, a study by Shu et al., did not apply blinding, thus triggering performance bias, detection bias, and attrition bias.21

Results showed that a study by Shu et al., did not apply blinding of the subject and personnel, thus triggering performance bias, detection bias, and attrition bias.
| Studies | Pre-intervention | Inter-vention | Post-intervention | Overall risk of bias | ||||
|---|---|---|---|---|---|---|---|---|
| Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | ||
| Leng et al., 2020.18 | Mod | Mod | Low | Low | Low | Low | Low | Mod* |
| Meng et al., 2020.14 | Low | Low | Low | Low | Low | Low | Low | Low |
*Mod: moderate. A study by Leng et al., 202018 had moderate quality because there was no difference in the analysis of group characteristics. This might lead to confounding bias.
Four out of five studies reported mortality within 28 days after MSC therapy. Lanzoni et al., reported mortality incidence in one in 11 patients treated with MSC, and seven in 12 patients who were given standard therapy.21 This led to a statistically significant difference in patient mortality rates between groups, with an OR of 0.07 [CI 95%: 0.01, 0.75]. Leng et al., and Shu et al., did not report any mortality in the intervention group.18,21 Meanwhile, Shi et al., did not report any mortality in two groups.19
Compilation of the study findings was conducted using a forest plot. The pooled OR for mortality risk for patients with MSC therapy was 0.13 [95% CI: 0.02, 0.68]. This value was obtained by the Mantel-Haenszel fixed effect model method because the heterogeneity was 0% and p value for heterogeneity was 0.76 (Figure 3).
Adverse event is a parameter that could represent the safety of a product. The adverse events referred to were urticaria, palpitations, or pulmonary edema (within six hours) and/or cardiac arrest (within 24 hours) after MSC administration or placebo or MSC vehicle (without MSC). All studies reported this parameter in their articles. None of these studies reported a significant difference in adverse event incidence between groups. Meng et al., and Shu et al., found no adverse events in either group.14,21
With the Mantel-Haenszel fixed effect model method, it was found that the pooled OR for the MSC group that experienced adverse events was 0.91 [CI 95%: 0.45, 1.86], with a heterogeneity value of 51% (Figure 4).
The need for a mechanical ventilator could represent the respiratory distress severity of a patient. In this systematic review, three out of five studies reported the need for a mechanical ventilator post-MSC therapy during the treatment period. None of the three studies reported a significant difference in the number of mechanical ventilators needed between groups. Only Shi et al., reported a higher percentage of mechanical ventilator use in the MSC group.19
In this study, pooled OR for the MSC group that required a mechanical ventilator during treatment was 0.42 [95% CI: 0.12, 1.47]. This figure was obtained by Mantel-Haenszel fixed effect model, with a heterogeneity of 0% (Figure 5).
There were only two studies reporting hospitalization duration. Meng et al. reported that the mean hospitalization duration in patients on MSC therapy was 20.5 ± 2.0207 days, and 23.25 ± 2.0361 days for patients without MSC administration.14 Shu et al., also agreed that the mean hospitalization duration in patients on MSC therapy was shorter than patients who received standard therapy, with a mean difference of 4.00 [−5.44, −2.56] days.21 The results of meta-analysis using the Mantel-Haenszel fixed effect model method found that the pooled mean difference was −3.54 [CI 95%: −4.68, −2.40] with 7% heterogeneity (Figure 6).
Leng et al., found a significantly greater increase in the IL-10 ratio and decrease in TNF-α in the intervention group compared to the control group within 10 days post-MSC administration.18 Meng et al., reported a significant reduction in IL-6 levels in patients receiving MSC therapy.14 Changes in IL-6 levels were not found to be significant in patients on standard therapy. Lanzoni et al., found a significant decrease in various inflammatory markers at six days post-MSC administration.20 Inflammation markers that were found to be significantly decreased were TNF-α, IL-2, IL-6, and IL-7. There was no significant decrease in these inflammatory markers in the control group. Shu et al., reported significantly lower levels of CRP and IL-6 in the MSC group compared to the control group at days three and seven post-MSC administration.21 On the same day, oxygenation index and lymphocyte count were found to be significantly higher in the MSC group.21
This systematic review and meta-analysis included five interventional studies. We assessed the heterogeneity of each outcome with the parameter I2 value in percent (%). Heterogeneity was noted to be quite low in all outcomes, except for the side effect outcome, which had moderate heterogeneity (51%). Moderate heterogeneity of side effects may result from different definitions or types of side effect reported in each study. Using a funnel plot, we noted that there was no significant risk of publication bias in all outcomes (Figure 7). The studies are evenly distributed in the right and left areas of the triangle forming a symmetrical funnel plot.
This meta-analysis attempted to compare MSC administration safety and efficacy in COVID-19 patients, which were assessed by mortality, adverse events, treatment duration, need for mechanical ventilators, and inflammation markers. To answer the formulated clinical questions, we used five interventional studies, two of which did not apply randomization. For the most part, the studies included in this meta-analysis reported that MSC was effective and safe to be given to COVID-19 patients.
We found that MSC administration was successful in reducing hospitalization duration and significantly reduced mortality risk. The decrease in hospitalization duration was certainly related to disease resolution. At hematological level, we also found a significantly greater increase in IL-10 ratio and decrease in TNF-α in MSC treated patients within 10 days post-MSC administration.19 IL-10 is an anti-inflammatory mediator that counteracts TNF-α action. Increased IL-10 and decreased TNF-α levels indicate cytokine storm resolution. Significant decrease in TNF-α, IL-2, IL-6, and IL-7 levels in MSC treated patients was also reported in studies that were included in this systematic review.21–23
COVID-19 pathogenesis could not be separated from immunity dysregulation, which manifests as a cytokine storm. COVID-19 triggers immunity dysregulation by triggering pro-inflammatory cytokine production cascade activation, mainly IFN-I.24 Other cytokines such as IL-1, IL-2, IL-4, IL-7, IL-10, IL-12, IL-13, IL-17, macrophage colony-stimulating factor (MCSF), granulocyte colony-stimulating factor (G-CSF), MCP-1, MIP-1α, interferon gamma-induced protein-10 (IP-10), IFN -γ, TNF-α, and hepatocyte growth factor (HGF) have also been shown to be increased in COVID-19 patients, resulting in cytokine storms and a worsening of the patient's condition.25 This systematic review indicates that MSC administration plays a role in improving COVID-19 patient’s condition through improvement of immunological status by decreasing pro-inflammatory cytokines.
Findings regarding MSC’s role in restoring immunological control in COVID-19 patients are supported by several studies that have proven beneficial effects. MSCs were shown to reduce TNF-α, IL-1 and IL-6 through hepatocyte growth factor (HGF), prostaglandin-E2 (PGE2), lipoxin A4-stimulated gene 6-protein (LXA4), TNF (TSG-6) release and suppression of inflammatory T-cell proliferation by indoleamine 2,3-dioxygenase expression, transition of Th1 and Th17 responses to Th2, and monocyte and mature myeloid dendritic cell inhibition.26
Treatment with MSCs is a therapeutic strategy to stop the uncontrolled inflammatory cascade and, at the same time, reduce post-COVID-19 pulmonary fibrosis and abnormal lung function, decrease ground-glass opacification (GGO) and infiltrate zones in patients post-MSC administration. This indicates that MSC therapy does not only improve patient’s immunological status, but also plays a role in structural improvement and prevention of lung damage in COVID-19 patients.27,28 These findings are supported by an in vivo study, which found that MSCs interfere with pulmonary fibrosis activation pathways resulting in pulmonary protective effects against damage/injury.29
Evidence that MSCs play a role in the immunomodulation process and lung structural repair supports the results that we found in this meta-analysis and systematic review, where MSCs effectively reduce mortality risk and reduce hospitalization duration. MSCs modulate oxidative stress, suppress systemic inflammation, and reduce lung damage progression.30
In addition, this meta-analysis also found that MSCs are safe to administer to COVID-19 patients. We noted no significant difference in adverse events incidence between groups. MSCs are a heterogeneous population of non-hematopoietic multipotent stromal cells with a specific cell surface expression pattern, low alloreactivity (expression of major histocompatibility complex (MHC)-I, MHC-II, and low co-stimulation molecules), and have the ability to differentiate tissues from favorable mesodermal lineage.31 Through these mechanisms, the body's resistance to MSC administration will be minimal. This confirms the findings of this meta-analysis that there are no adverse events in patients after MSC administration, and MSCs are safe to administer to COVID-19 patients.
From our knowledge, there has been no meta-analysis or systematic review addressing the clinical questions explored in this article. Apart from the clinical questions that were successfully answered through this meta-analysis, there are several limitations that need to be underlined. First, the exclusion criteria were not applied for the MSC’s type of source and seeding methods. Second, all comorbid patients were included. Patients with degenerative or malignant diseases were perceived to have different immunological responses, which might affect MSC therapy efficacy. Third, some studies have low quality or a fairly high bias risk, especially those that did not apply blinding to their studies. In addition, the number of participants in each study and overall is still considered insufficient.
Giving MSCs to COVID-19 patients is safe and effective in reducing mortality and hospitalization duration. Clinical improvement in patients occurred through cytokine storm resolution, which was characterized by decreased pro-inflammatory cytokines and increased anti-inflammatory suppression in patients.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: PRISMA checklist for ‘Mesenchymal stem cell therapy efficacy in COVID-19 patients: A systematic review and meta-analysis’, https://doi.org/10.6084/m9.figshare.16602203.v1.16
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This work was supported by Indonesian Endowment Fund for Education (LPDP) Republic of Indonesia.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
References
1. Basiri A, Mansouri F, Azari A, Ranjbarvan P, et al.: Stem Cell Therapy Potency in Personalizing Severe COVID-19 Treatment.Stem Cell Rev Rep. 17 (1): 193-213 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Stem cell research, Cell-based therapy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 22 Sep 21 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)