Keywords
Anti-Mullerian hormone, ART, infertility, ovarian stimulation protocols, blastulation rate, oocyte retrieval, embryo utilization rate, fertilization rate
Anti-Mullerian hormone, ART, infertility, ovarian stimulation protocols, blastulation rate, oocyte retrieval, embryo utilization rate, fertilization rate
Infertility is a clinical condition, in which there is a failure to achieve pregnancy even after unprotected intercourse for a period of 12 months. Globally, the prevalence of infertility is estimated at 8–12% for women aged 20–44 years old, with 1 in 6 couples experiencing some form of infertility problems (ART fact sheet, European Society of Human Reproduction and Embryology). Recently, in Malaysia the total fertility rate of women of reproductive age in Malaysia has declined to 1.7 babies in 2020 as compared to 1.8 in 2019.1 One of the hallmark causes of infertility in the majority of the population is delaying conception to the late thirties or in early forties.2 Physiological ovarian aging is referred to as an age-mediated decrease in functional ovarian reserve within expected ranges. Previous reports have shown that female ovarian reserves decrease with substantial increases in age. The ability of fertilization during natural and stimulated ovarian cycles declines with maternal age, and thus optimal fertility is achieved in the age range between 20–30 years old.3 Globally, recent socio-economic changes have led to increases in infertility rates and there have also been increased numbers of women approaching for assisted reproductive technology (ART) treatments. While mounting treatment strategies are employed in the management of infertility, such as intrauterine insemination (IUI), to in vitro fertilization (IVF), intra-cytoplasmatic sperm injection (ICSI), testicular sperm extraction (TESE) and preimplantation genetic testing (PGT), ART still remains the gold standard for controlled ovarian stimulation.4
Various markers of ovarian reserve, which determines the quantity of primordial follicles and oocyte quality, have been established as predictors of ovarian response.5 Among the biochemical parameters, such as basal follicle stimulating hormone (FSH), estradiol and inhibin levels may differ during the menstrual cycle and thus their diagnostic utility is limited.6 Imaging modality using transvaginal ultrasonography is used to evaluate the antral follicle count (AFC) but its sensitivity is affected by sonographers' intra- and inter-observer reproducibility.7
Anti-Müllerian hormone (AMH), a dimeric glycoprotein that belongs to the transforming growth factor-β (TGF-β) family, has been determined as an effective surrogate marker in the evaluation of functional ovarian follicle reserve.8 AMH is synthesized by granulosa cells of the preantral and small antral follicles and also serves as a follicular gatekeeper by blocking the initial follicle recruitment and FSH-mediated growth and selection.9 When compared with other ovarian reserve biomarkers, AMH displays high sensitivity for stability and good measurement repeatability. Earlier reports have shown the diagnostic accuracy of AMH levels in the prediction of high and poor responses during gonadotropin-releasing hormone (GnRH) agonist or GnRH antagonist protocols10,11 and also in AMH-mediated ovarian stimulation.11 Safe and effective ovarian stimulation is a pivotal step in the success of ART treatment cycles. It is important to categorize patients who plan to undertake ART as normal, poor or high responders, thus choosing the appropriate dosage of gonadotropin for every patient that could yield a suitable number of oocytes.12 In this backdrop, the present study was performed to evaluate the FSH and Human Menopausal Gonadotropin (HMG) dosages on the outcomes of ART in low, normal and high responders.
A total of 191 women aged 18–45 years old who were undergoing ART were recruited at TMC Fertility and Women’s Specialist Centre, Puchong, Selangor, Malaysia using a convenience sampling method. This study was approved by the Ethics Committee of Thomson Hospital Kota Damansara, Malaysia in January 2020 (ref no. THKD/GMA/EC/0622/001). TMC Fertility & Women’s Specialist Centre is a branch and subsidiary of Thomson Hospital, hence all ethical clearance for research and studies must be from the Thomson Hospital, Kota Damansara Ethics Committee. Written informed consent was obtained from all the patients and it was documented in all their case notes. The study was conducted between March and May 2022.
The population of the study included female patients ranging between the ages of 18–45 years old.
Patients undergoing cancer therapy, oocyte donors and patients on immune suppressant drugs were excluded from this study.
Blood samples were collected within 12 months on commencement of the treatment. The blood samples were centrifuged at 3,000 rpm for 10 min to obtain the blood serum.
AMH levels were measured using Electrochemiluminescence Immunoassay (ECLIA) kit (Roche) (Catalogue number: 06331076190) according to the manufacturer’s instructions mentioned in the protocol at the Gribbles Pathology Laboratory in Petaling Jaya, Selangor, Malaysia. According to the AMH levels, the patients were classified as low responders (AMH ≤ 5.4 pmol/L), normal responders (5.5–24.9 pmol/L), and high responders (≥25 pmol/L) based on the guidelines by the National Institute of Health and Care Excellence (NICE).13
Ovarian stimulation was done by using various combinations of FSH market preparations. It was either Puregon by MSD, Gonal F by Merck Serono or Folliculin by Bharat Serums. The HMG used in the study was either from Humog by Bharat Serums or Menopur by Ferring. The stimulation protocol was performed by decreasing the pituitary levels using GnRH agonist (long stimulation) or by GnRH antagonist to restrict the premature ovulation. Ultrasound follicular tracking was used to determine the doses. To avoid hyperstimulation, when a minimum of two follicles were 17 mm in diameter or one follicle had a diameter higher than 18 mm were detected, HCH 5,000 or 10,000 IU 0.2 mg Decapeptyl was administered for the final oocyte maturation. After 35 to 36 hours of hCG or agonist administration, the oocytes were retrieved under vaginal ultrasound-guided ovarian needle puncture. Among the study participants who were considered as high risk for ovarian hyperstimulation syndrome (OHSS), the embryos were cryopreserved. The physician decision to extend the embryo culture to five days rather than the routine culture of three days was dependent on the number of embryos with good morphological quality at day three. Vaginal micronized progesterone was given for luteal support until 12 weeks of pregnancy. FSH and HMG dosages in controlled ovarian stimulation were determined. Retrieval, maturation, fertilization and utilization rates were measured.
The data were represented as mean±SD. Descriptive analysis was done for the demographics and clinical characteristics. Subgroup analysis was done using one-way ANOVA followed by Tukey’s post hoc analysis between the groups. The correlation between AMH levels and different fertilization variables was determined using Pearson’s correlation analysis. The data were analyzed using IBM SPSS Statistics software version 24 (RRID:SCR_016479). P<0.05 was considered as statistically significant. The missing data were omitted and not included in the final analysis
This prospective study was conducted on 191 patients who met the inclusion criteria. The basic demographics and clinical characteristic of the participants are shown in Table 1.27
Regarding age distribution, the majority of the participants were aged between 30–35 years old (40.8%), followed by 36–40 years old (34%). The results are shown in Table 2.
| Age range | n (%) |
|---|---|
| Age, years | |
| Less than 20 | 1 (0.5%) |
| 20–25 | 5 (2.6%) |
| 26–30 | 12 (6.3%) |
| 30–35 | 78 (40.8%) |
| 36–40 | 65 (34%) |
| 41–45 | 30 (15.7%) |
| Total | 191 (100%) |
AMH distribution among the study participants is shown in Table 3. According to the AMH levels, the majority of the patients were normal responders (AMH:5.5–24.9 pmol/L), which constituted 52.4% of patients, high responders (AMH: ≥ 25 pmol/L) encompassing 29.8% of patients and low responders (AMH ≤ 5.4 pmol/L), which constituted 17.8% of patients.
| Category | AMH levels, pmol/L | Frequency | Percentage, % |
|---|---|---|---|
| Low responders | ≤5.4 | 34 | 17.8 |
| Normal responders | 5.5–24.9 | 100 | 52.4 |
| High responders | ≥25 | 57 | 29.8 |
Subgroup analysis using ANOVA showed that average FSH per day (p=0.01) and average HMG per day (p=0.002) among low, normal and high responders were significantly different, with low responders needing higher amounts of HMG and FSH per day than high responders. Also, high responders needed a fewer number of days of HMG than low responders and the difference was statistically significant. The number of oocytes retrieved was statistically significant (p=0.001) among different responders. The number of oocytes retrieved among high responders was comparatively more than low responders. The retrieval rate was statistically significant (p=0.001) among different responders. The retrieval rate among high responders was comparatively higher than low responders. The blastulation rate was statistically significant (p=0.02) among different responders. The retrieval rate among high responders was comparatively higher than low responders. However, rates of maturation (p=0.45), fertilization (p=0.07) and embryo utilization (p=0.51) were not statistically significant among the groups. The results are shown in Table 4.
| Variables | Mean±SD (Total n=191) | P-value |
|---|---|---|
| Avg FSH per day, IU | ||
| Low responders (AMH ≤ 5.4 pmol/L) | 236.2±121.5 | 0.017* |
| Normal responders (5.5–24.9 pmol/L) | 278.0±284.9 | |
| High responders (≥25 pmol/L) | 175.6±57.1 | |
| Days of FSH | ||
| Low responders | 9.6±4.3 | 0.101NS |
| Normal responders | 10.8±1.9 | |
| High responders | 10.4±2.8 | |
| Days of HMG | ||
| Low responders | 6.4±4.9 | 0.002* |
| Normal responders | 5.6±4.5 | |
| High responders | 3.3±4.2 | |
| Avg HMG per day, IU | ||
| Low responders | 136.7±133.3 | 0.002* |
| Normal responders | 101.9±80.7 | |
| High responders | 62.4±96.3 | |
| Oocytes retreived | ||
| Low responders | 4.3±3.1 | 0.001* |
| Normal responders | 13.9±8.3 | |
| High responders | 20.9±10.9 | |
| Retrival rate, % | ||
| Low responders | 67.9±32.8 | 0.001* |
| Normal responders | 88.1±19.3 | |
| high responders | 89.7±18.0 | |
| Maturation rate, % | ||
| Low responders | 75.5±34.0 | 0.456NS |
| Normal responders | 74.5±17.2 | |
| High responders | 79.3±23.7 | |
| Fertilization rate, % | ||
| Low responders | 55.9±36.8 | 0.074NS |
| Normal responders | 67.6±23.8 | |
| High responders | 62.8±22.9 | |
| Blastulation rate, % | ||
| Low responders | 40.4±42.8 | 0.02* |
| Normal responders | 57.9±29.4 | |
| High responders | 50.6±34.6 | |
| Embryo utilization rate, % | ||
| Low responders | 46.7±36.4 | 0.510NS |
| Normal responders | 48.0±27.2 | |
| High responders | 53.9±28.7 |
A positive correlation (r=0.617) was found between AMH and number of oocytes retrieved, which was statistically significant (p=0.01). Likewise, a positive correlation (r=0.545) was found between AMH and number of oocytes sucessfully fertilized and the association was statistically significant (p=0.01). However, the corelation found between AMH levels and number of embryos utilized was not statistically significant (r=0.08; p=0.23). The results are shown in Figure 1 and Figure 2.
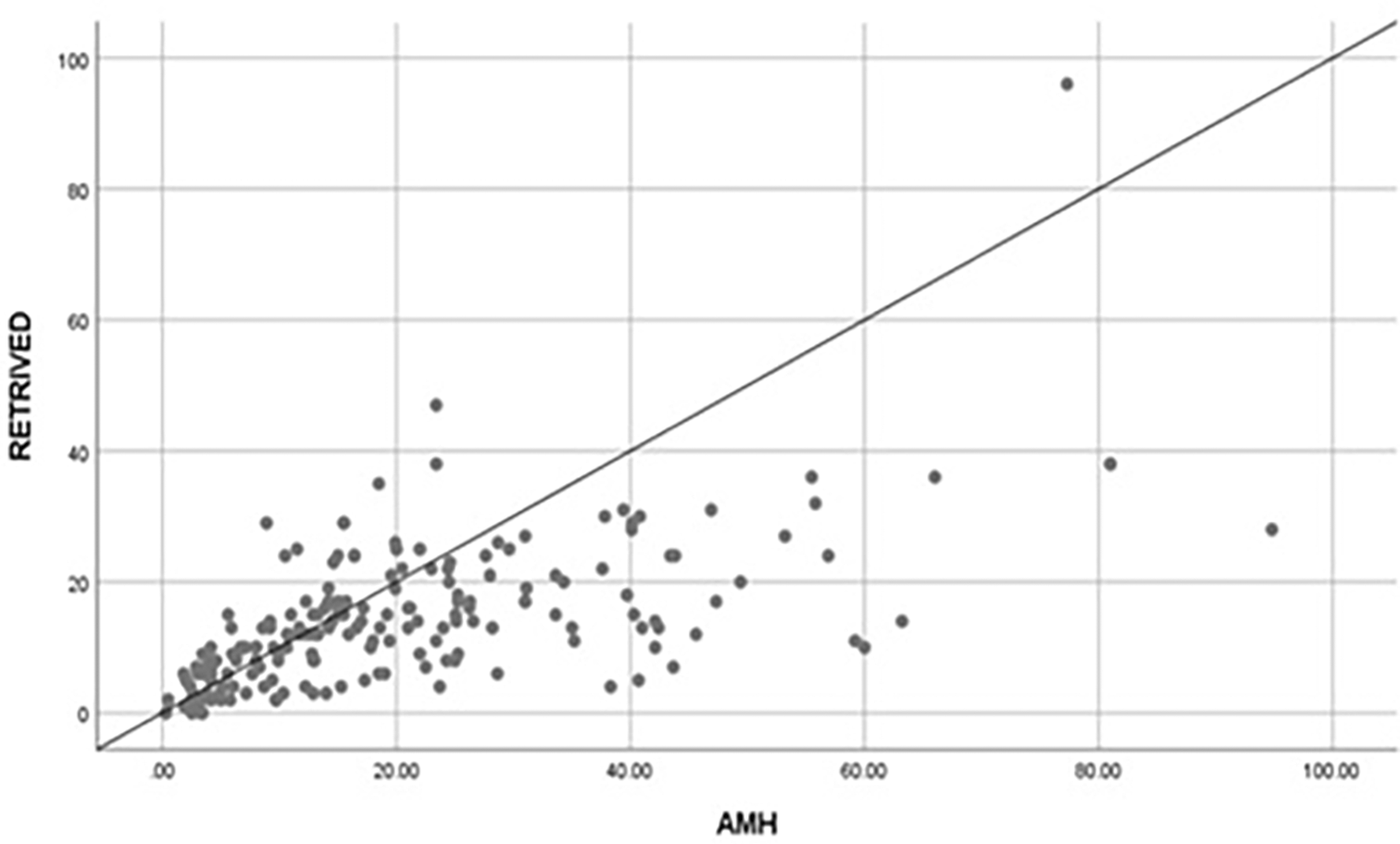
AMH, anti-Müllerian hormone.
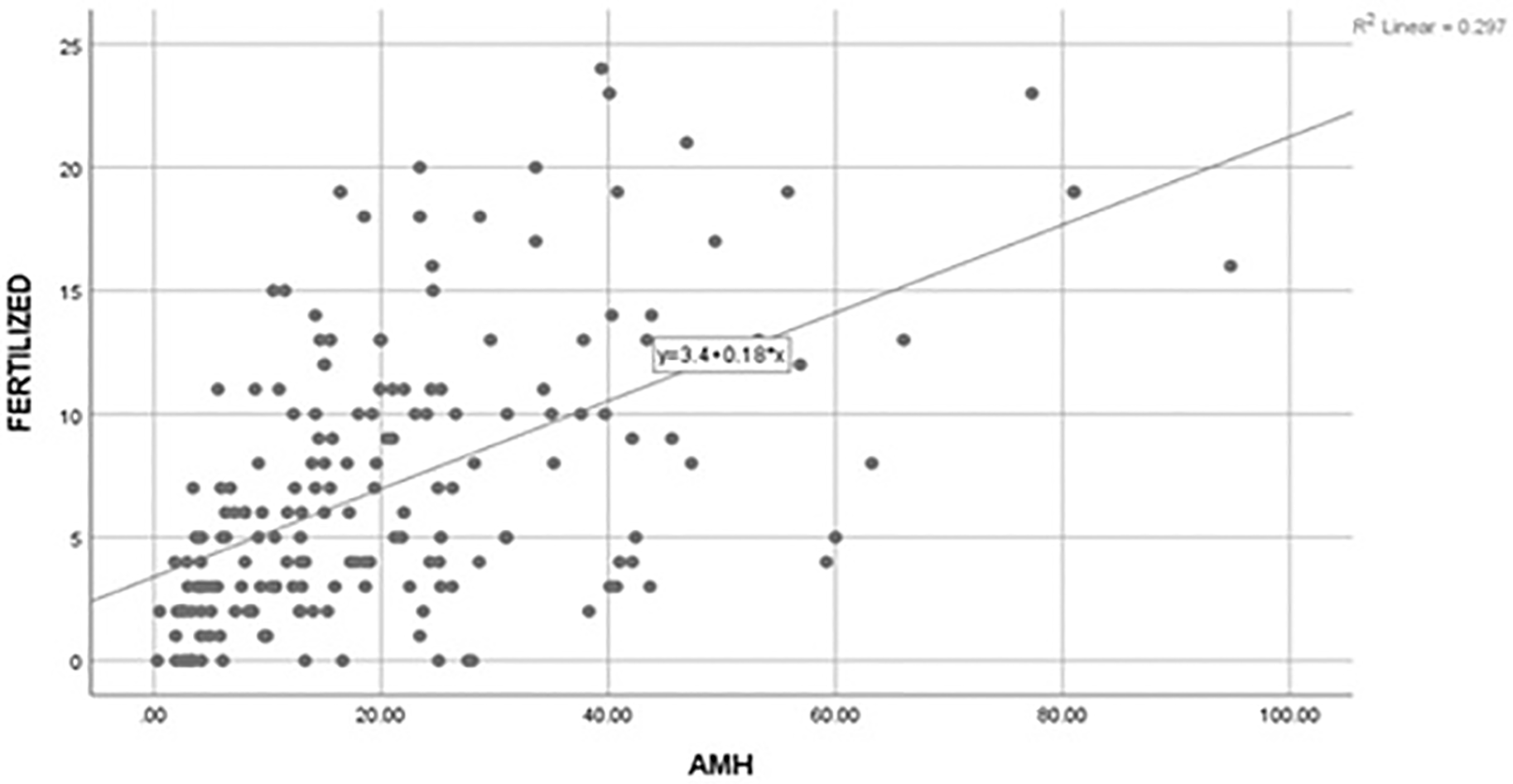
AMH, anti-Müllerian hormone.
Association between age and AMH levels are shown in Figure 3. A negative correlation (r=-0.391) exists between age and AMH levels (as age increases AMH levels decrease) and the association was found to be significant (p=0.01).
The present study confirmed the usefulness of AMH as a reliable biomarker of ovarian function. A mounting number of studies have shown that AMH is a new technique for the evaluation of ovarian reserve. AMH levels decrease throughout a woman’s reproductive life.14 Serum AMH levels on day three of the menstrual cycle display marked reduction with age and show marked association with AFC. During spontaneous menopause, undetectable AMH concentration have been observed.15 In women with normal menstrual phase, oophorectomy leads to the disappearance of AMH in three to five days, and thus substantiates that circulating AMH is attributed to ovarian origin.16 AMH is a reliable endocrine marker, which provides information about the transition of resting primordial follicles to growing follicles. AMH levels show a decreasing pattern five years before the last menstrual cycle and thus showcases the biological event of menopause.17
In the present study, based on the AMH levels the patients were categorized as low, normal and high responders. We observed a high prevalence of normal responders, with 52.4% as compared to high responders (29.8%) and low responders (17.8%). In the current study, the average FSH per day was significantly higher in low responders as compared to high and normal responders. Earlier trials showed that the oocyte yield can be upregulated using higher doses of FSH,18,19 thus in routine clinical practice, the low responders must be treated with higher doses as compared to the standard FSH dose of 150 IU/day (from 225 to 600 IU/day) to increase the number of retrieved oocytes. Similarly, the average HMG per day was significantly higher in low responders as compared to high and normal responders. There has been no marked difference between HMG and FSH for ovarian stimulation with respect to intermediate outcome and ART surrogate markers. Further for both HMG and FSH, the outcomes are similar for Metaphase II (MII) matured oocytes, frequency of high-quality embryos, zona pellucida morphology, and polar body evaluation.20 Previous reports have shown that there are no marked effects in the number of oocytes retrieved with HMG, however mounting studies indicate that there has been minimal decrease in the number of oocytes retrieved (typically around one oocyte less per retrieval).21
In the present study, oocytes retrieved, retrieval rate and blastulation rate were significantly higher in high responders compared with low responders. To some extent, the results shown were anticipated as AMH has been used an indicator of oocyte reserve in previous studies, whereas the resulting fertilized or transferred embryos may be due to a chance process based on many various factors such as quality of oocyte and sperm.22 Furthermore, in our study there were no significant differences in the maturation rates, fertilization rates and embryo utilization rates among the low, normal and high responders. Similarly in a study conducted by Umarsingh et al.,23 there was no statistically significant relationship between numbers of oocytes fertilized vs. AMH category (p>0.05).
In the present study, there was a positive significant relationship between AMH and number of oocytes retrived and number of oocytes sucessfully fertilized. Similar to the present study, Kotanidis et al.,24 reported that there was a strong, positive correlation between AMH and the number of collected oocytes. Furthermore, Wiweko et al.,25 also reported that serum AMH levelsarethe signficant predictor of oocyte quality. Meanwhile, we have observed non-significant associationsbetween AMH levels and the number of embryos utilized. Our study corroborates with the findings of Umarsingh et al.,23 where there was no signficant relationship between AMH and embryo utilization.
The Pearson’s correlation test found an R-value of -0.328 between AMH and age, thus showing that there was a negative association, which was found to be significant (p=0.01). A stronger relationship between these two variables was expected as it is known that as age increases, AMH should decrease. Also, itis suggested that serum AMH is identified as the improved endocrine marker to measure the reproductive capability in advanced age. Similarly, Ishii et al.,26 reported an inverse moderate correlation (correlation coefficient r=-0.5, p<0.01) between age and AMH levels.
In conclusion, the study outcome revealed that AMH serves as a reliable marker to evaluate ovarian reserve and may benefit women with advanced age planning for pregnancy. Albeit AMH hormone serves as an effective endocrine marker however, its valuable role in the assessment of decline in the ovarian pool must be compared with other markers. AMH can also significantly predict the number of oocytes retrieved and number of oocytes sucessfully fertilized. Further, the study findings also confirmed that age displayed signficant assocaition with AMH levels. In addition, the rates of retrieval, maturation, fertilization, embryo utilization and blast were higher among the high responders as compared to low responders. Thus, increase in dosages of ovarian stimulation protocol is required among the low responders to achieve quality embryos. During counseling, the level of AMH is important to educate patients regarding the high dose of medication administration in order to achieve a good outcome.
Dryad: Evaluation of assisted reproductive technology treatment outcomes based on stimulation dosages and anti-mullerian hormone levels. https://doi.org/10.5061/dryad.gxd2547pr.27
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Reproductive endocrinology and infertility
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 12 Sep 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)