Keywords
Heart failure, Sarcopenia, Depression, Functional classification, Prevalence
Heart failure, Sarcopenia, Depression, Functional classification, Prevalence
A global increase in heart failure (HF) has been reported in several developed and developing countries. Research indicated that the number of patients with HF has been increasing from 33.5 million in 1990 to 64.3 million in 2017.1 In addition, the incidence of HF is increasing for individuals with advanced age2 and increasing age-related symptoms of HF and higher number of HF in ages of ≥ 70 years.1 Lippi and Sanchis-Gomar3 estimated that the prevalence of overall HF is projected to be 50% by the year 2030 in low to middle socio-demographic index regions.3
Causes of HF are described as abnormalities of cardiac function or cardiac structure that lead to inadequate cardiac output.2 The factors that contribute to the development of sarcopenia in HF are physical inactivity, low muscle blood flow, hormonal changes, and endothelial dysfunction.4 These factors affect the reduction in muscle mass and muscle strength, including low physical performance. As a result, patients with HF display symptoms of depression and poor quality of life.
Recently, a systematic review and meta-analysis (from 11 articles on 1742 patients with HF) showed a 10% to 69% prevalence of sarcopenia in HF patients.5 Furthermore, the prevalence of sarcopenia in patients who were hospitalized with HF was reported to be 55%, whereas those who were still ambulatory was 26%.5
Regarding symptoms of depression in HF patients, the prevalence of depressive symptoms appears to be 20%.3 However, the variation in the prevalence ranged from 9%-60%, depending on measurement and population.6 In addition, depression is often related to adverse health events. Evidence found that a decrease in muscle mass was noted in patients with depression.7,8 Additionally, symptoms of depression in sarcopenia were identified to be relatively high, indicating a relationship between sarcopenia and depression.9 A higher prevalence rate of depression among individuals with sarcopenia compared to the general population has also been identified.9 However, few studies have reported depression in HF patients with sarcopenia. Therefore, this study aimed to explore the prevalence of depression among HF patients with sarcopenia and sarcopenia’s relationship with depression in HF patients.
A cross-sectional study was designed with both male and female HF patients at Heart Failure Clinic Thammasat University Hospital and Center of Chest Institution in Thailand. The sample size was calculated based on the study by Canteri et al.,10 the prevalence of sarcopenia in HF was 10.1%; therefore, the sample size was 140. To prevent insufficient data, 154 participants were enrolled in the present study.
An information sheet and consent form were given to all participants. The protocol has been approved by the Human Research Ethics Committee of Thammasat University (Science), Thailand in accordance with the compliance with the Declaration of Helsinki, the Belmont report, the Council for International Organizations of Medical Sciences (CIOMS) guidelines and the International Practice (ICH-GCP), COA no. 071/2563.
All male and female participants aged 35 years or older, who were receiving medical treatment due to HF were enrolled. Participants who had unstable angina, uncontrolled arrhythmia, blood pressure greater than 180/100 mmHg at rest, resting heart rate higher than 120 beats per min, exacerbated chronic obstructive pulmonary disease (COPD), or musculoskeletal or neurological health problems that affect the tests were excluded.
Based on the Asian Working Group for Sarcopenia (AWGS) diagnosis criteria in 2019, sarcopenia is defined as poor physical performance and/or muscle strength as well as low muscle mass; individuals who exhibit all of these criteria are categorized as having severe sarcopenia.11 In addition, possible sarcopenia is defined as low muscle strength with or without decreased physical performance. Poor physical performance was examined utilizing a 6-meter walk test to determine gait speed, and the cut-off value of <1.0 m/s was categorized as a slow gait speed.11 Poor muscle strength was measured by utilizing a handgrip dynamometer dynamometer (T.K.K. 5401; Grip-D; Tokyo, Japan); the cut-off value was less than 28 kg for men and less than 18 kg for women.11 The participants were asked to perform the handgrip dynamometer test twice with their dominant hand while standing, and the average hand grip strength was reported. Low muscle mass was evaluated by using bioimpedance analysis (HBF-375, Omron Healthcare Co., Ltd., Japan), skeletal muscle mass (SMM) was calculated by skeletal muscle percentage multiplied with body weight (kilogram); therefore, skeletal muscle index (SMI) was calculated by SMM in kg adjusted for the squared height (SMM/hight,2 kg/m2). According to AWGS 2019, a SMI value of <7.0 kg/m2 in men and <5.7 kg/m2 in women was defined as low muscle mass.11 This method has previously been utilized and published elsewhere.12 All participants were evaluated by a trained physical therapist.
The Patients Health Questionnaire 9 (PHQ-9) is widely used for identification of depressive symptoms.13,14 The Thai version of PHQ-9 with a cut-off score of ≥ 9 has shown a good sensitivity and internal consistency (0.84 and 0.77, respectively).15 All participants were asked to complete the PHQ-9 for depressive symptom screening.
ANOVA, T-test, and chi-square tests were used for the comparison between sarcopenia and no-sarcopenia or depression and no depression, where appropriate. The relationship with depression was analyzed by Pearson correlation or Spearman rank correlation, and p-value was set at <.05.
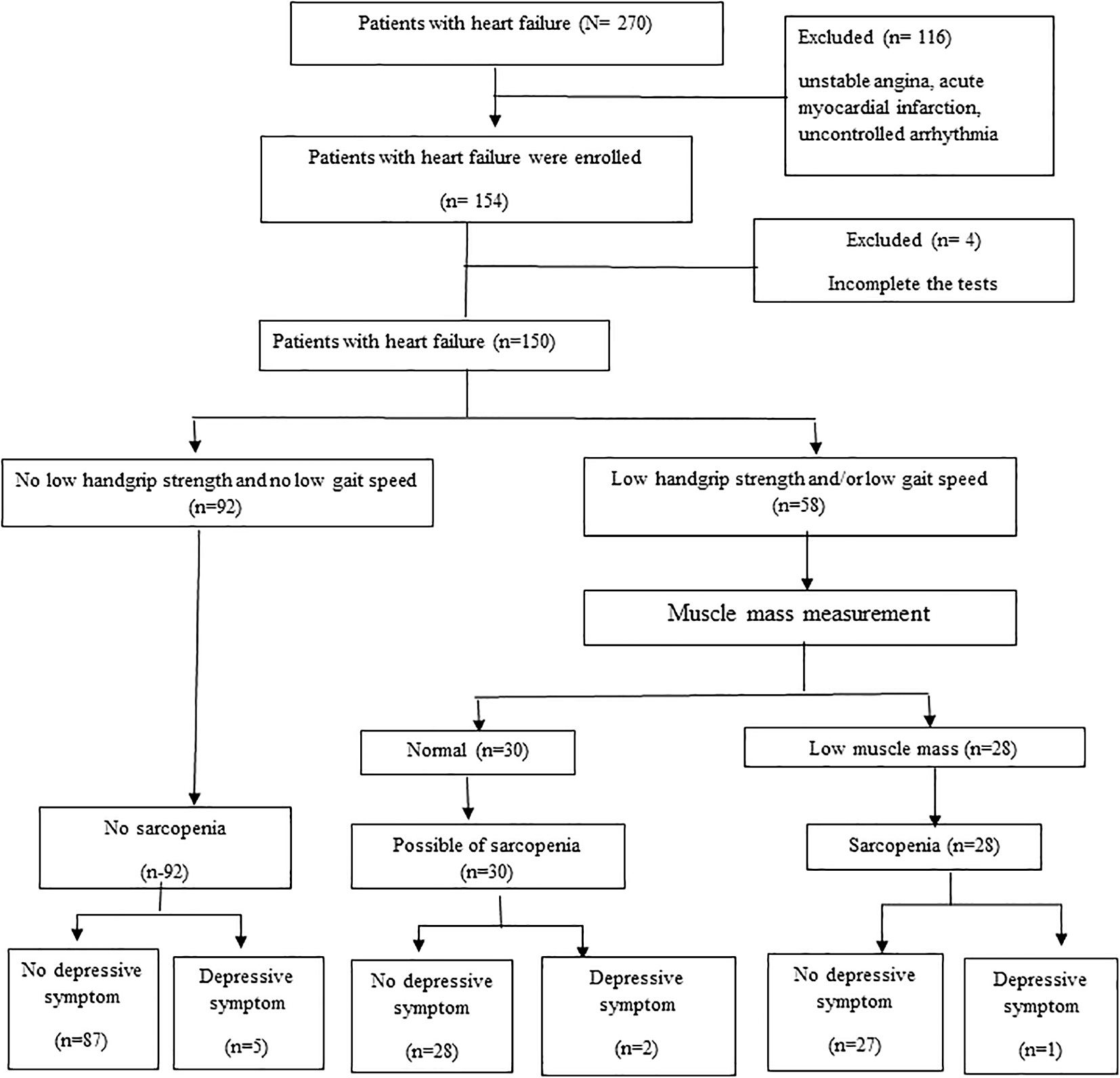
Of the 154 participants, only four individuals did not complete the tests (e.g., depression questionnaire or sarcopenia screening). Finally, 150 people with HF were included in the analysis and the prevalence of sarcopenia was 18.67% (i.e., seven (4.67 %) in severe sarcopenia and 21 (14.00%) in sarcopenia) (see Figure 1).
Statistically significant differences were observed in age, sex, body mass index (BMI), and left ventricular ejection fraction (LVEF) between sarcopenia and no sarcopenia in HF patients. Compared to participants with no sarcopenia, those with sarcopenia had an older age, low BMI, low LVEF, and found in female gender. However, no significant differences between depression scores (i.e., PHQ-9) and New York Heart Association (NYHA) functional classification in sarcopenia and no sarcopenia were observed in HF patients (see Table 1).
Therefore, the PHQ-9 was performed to define depression in patients with HF using cut-off scores of nine categorized depression symptoms.15 Chi-square test revealed that eight out of 150 (5.33%) participants were categorized as having depression. In addition, only one HF patient with sarcopenia out of 28 (3.57%) fell into the depression category. Furthermore, higher depression scores were related to the NYHA functional class (r= 0.319, p<.001); indicating that high depression scores reduced functional classification (see Table 2).
| Sex # (p-value) | Age (p-value) | BMI (p-value) | EF (p-value) | NYHA# (p-value) | SMI (p-value) | HGS (p-value) | GS (p-value) | |
|---|---|---|---|---|---|---|---|---|
| PHQ-9 | 0.113 (.169) | 0.153 (.062) | -0.140 (.087) | -0.080 (.330) | 0.319 (<.001) | -0.047 (.569) | -0.023 (.781) | -0.101 (.217) |
An ANOVA was then conducted to compare between NYHA functional classification and depression scores. Analysis indicated that individuals with NYHA functional class I (defined as having no symptoms and no limitations in ordinary physical activity) had lower PHQ-scores than those with NYHA functional class II (i.e., mild symptoms and slight limitations during ordinary activity) and NYHA functional class III (marked limitations in activity due to symptoms, only comfortable at rest) with the scores was 1.23±0.22, 2.30±0.41, and 5.44±1.20, respectively; F=13.979, p<.001 (see Figure 2).
The present study focused on the prevalence and relationship between sarcopenia and depression among patients with HF. Results indicated that the prevalence of sarcopenia was 18.6% according to the AWGS criteria in 2019, and the prevalence of depression (defined as PHQ-9) was 5.33%. In addition, depression in HF patients with sarcopenia was 3.57%. A relationship was only identified between depression and NYHA functional classification.
The prevalence of sarcopenia was 18.67% among HF patients, which corresponds with previous studies.10,16,17 Fulster et al.16 reported that 19.5% of patients with HF had sarcopenia. However, a systematic review with 11 studies reported that the average prevalence was 34%, ranging between 10% and 69% of those patients.5 In addition, several studies indicated that the factors associated with sarcopenia in patients with HF were sex, age, BMI, and LVEF. Konishi et al.18 reported that the prevalence of sarcopenia plays an important role in the mortality of HF patients with both reduced ejection fraction preserved and HF patients with preserved ejection fraction. Pathology of HF might be related to an inflammatory cytokine and lead to an imbalance between catabolism and anabolism, which results in impairment of skeletal muscle and function of the heart, in particular LVEF.19
Regarding depressive symptoms, this study used cut-off scores on the PHQ-9 (≥9).15 The prevalence of depression was only 5.33% among HF patients, which is a relatively low prevalence of depression. In contrast to other studies, the European Society of Cardiology reported that 20% of patients with HF exhibit depressive symptoms.2 Additionally, a meta-analysis of 36 studies reported that the prevalence of clinical depression among HF patients was approximately 21.5%.20 Of those, 33.6% of participants exhibited high depressive symptoms based on questionnaires and 19.3% indicated depression through diagnostic interviews.20 Patients with HF who were screened with questionnaires were identified as exhibiting much higher depressive symptoms compared with diagnosis from interviews or psychiatric diagnoses. In addition, a literature review on the prevalence of depression in congestive HF found that patients with congestive HF had a two to three times higher prevalence rate of depression than the general population.21 Participants with heart disease had a high risk of new onset depressive symptoms.22 Previous studies indicated a high prevalence of depression in HF patients, ranging between 17.4-51.1%.23–25 However, in the present study, the prevalence of depression in HF patients was identified as only being 5.3%; this might be due to problems of self-reported bias in the depression questionnaire. Several studies indicated that self-reported bias and underreporting of depression were based on gender differences, where males show a low score compared to females.26–28 In our study, females indicated higher depression scores (i.e., PHQ-9) than male participants with HF; however, statistical significance was not observed (data was not shown). It should also be noted that of the patients who were enrolled, 70.63% were male, whereas only one-third of female’s patients with HF were recruited. Therefore, the number of different gender participants may be conceptually problematic. Additionally, several studies reported that a short duration of HF indicated a higher rate of depression than those patients diagnosed with HF for a long time.24,25 However, the present study has not reported the duration of HF diagnosis. Hence, further studies should consider gender differences and record the duration of disease.
The present study also found that depression scores were related to the functional classification of NYHA; higher the depression scores, higher the severity of the functional class. In line with previous studies, a high prevalence of depressive symptoms in HF was observed in NYHA class IV compared to NYHA class I.20,29 Association of depression with functional class in heart disease was higher in NYHA class III/IV compared to NYHA class I and II.30 However, no relationship was identified between depression and sarcopenia in the present study. Several previous studies, which explored sarcopenia and depression indicated that individuals with sarcopenia had a high depression score or depressive symptoms.22,31–33 In the present study, it should be noted that low response rate to the PHQ-9 items in HF was observed (2.13±3.14 scores), which may have limited the ability to determine the increased risk for scoring high on sarcopenia.
Some limitations that might affect sarcopenia and depression in HF patients should be taken in consideration for future research. This study enrolled patients with clinically stable HF and ambulatory patients. In addition, over 70% of the recruited participants were male. Therefore, the prevalence of depressive symptoms in HF patients with sarcopenia might be underestimated due to the relatively small sample size and recruitment of stable patients. In addition, demographic data such as marital status and duration of disease were not reported in association with depression.34 To explore the prevalence of depression in individuals with sarcopenia, future studies might recruit people with sarcopenia or identify sarcopenia in people who are diagnosed with depression.
In conclusion, sarcopenia which was assessed using the AWGS 2019 criteria, was prevalent in 18.67% and only 5.33% of participants exhibited depressive symptoms. In addition, only 3.57% were identified as having both depression and sarcopenia. NYHA functional classification was related to depression.
Zenodo. Association between depression and sarcopenia in patients with heart failure. DOI: 10.5281/zenodo.7057739.35
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank Prof. Manote Lotrakul, MD., Department of Psychiatry, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand for his permission to use the Thai version of PHQ-9. We would also like to thank staff from Thammasat University Hospital and Central Chest Institution, Thailand, for their support. We gratefully to thank the participants and their caregivers for participating in the study. Finally, the authors gratefully acknowledge the financial support provided by the Faculty of Allied Health Sciences Research Fund, Contract No.7/2564, Thammasat University. There is no conflict of interest in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)