Keywords
Phase change materials, Capric acid, Paraffin, DSC
This article is included in the Uttaranchal University gateway.
This article is included in the International Conference on Clean Energy Systems and Technologies collection.
Phase change materials, Capric acid, Paraffin, DSC
When energy output is insufficient to meet demand, efficient use of existing energy is critical.1 Thermal energy may be stored in three ways, namely: thermochemical energy storage, sensible heat storage, and latent heat storage (LHS). Thermal energy is absorbed or released in a thermochemical heat storage method by using a completely reversible chemical mechanism to break and mend molecular bonds. In sensible heat storage, thermal energy is stored by heating a solid or liquid to the appropirate temperature. The LHS method relies on the absorption or release of heat during the solid-liquid transition, or liquid-gas transition, as a storage medium. Phase change materials (PCMs) are a type of LHS material that can store and release a significant amount of energy per unit mass.2 This energy transfer happens whenever there is a transition from solid to liquid or liquid to solid. This whole process is isothermal. Among all the listed methods, the LHS is the most appealing, and it is capable of compensating for the energy mismatch between the production and consumption of heat.3,4 There are different kinds of PCMs (organic, inorganic, eutectic) that can melt or solidify at a range of temperatures, and they can be used for a range of applications depending on the temperature. PCMs, find their application in energy-efficient buildings, industrial waste heat recovery, central air-conditioning systems, solar thermal energy storage, temperature-adapted greenhouses, and thermoregulating fibers.3,4
Among the PCMs investigated, fatty acids, paraffin wax (PW), and their mixtures have drawn a lot of attention. The reasons being that fatty acids have high latent heat of fusion (LHF), exhibit consistent melting, and freezing characteristics, as well as freezing without supercooling, low vapor pressure, non-toxic, minimal volume change during solid-liquid/liquid-solid transitions, self-nucleating characteristics, good chemical and thermal stability over an extended duration of usage, and easy availability.3,5 Animal and plant-derived fatty acids have the extra benefit of providing a constant supply, notwithstanding fuel shortages. Apart from that, fatty acids are abundant due to their bio-origin and are recyclable, eco-friendly due to lower carbon emissions, and essential for long-term growth.6 The major challenge of employing fatty acids as PCM is their pungent stench, corrosive nature, and notably their rapid sublimation rate.7 On the other hand, the advantage of using PW is that it is non-corrosive, safe, reliable, chemically stable, inert below 500 °C, has low vapor pressure in the melt form, minimal volume change during solid-liquid, and liquid-solid transition, and is less expensive as compared to other PCMs.3,8 PW has certain undesirable characteristics, such as being mildly flammable and incompatible with plastic containers.3,8 In his research, Kahwaji et al. looked at the thermophysical properties, thermal stability, and chemical compatibility of paraffin PCMs. PW48, PW52, and PW58 have melting temperature (Tm) values of 48.2 1.5 °C, 51.8 1.5 °C, and 57.7 1.5 °C, respectively. For the same, the LHF were 156 16 kJ/kg, 171 17 kJ/kg, and 197 20 kJ/kg, respectively. The thermal stability of paraffin PCMs was reported to be over 3000 thermal cycles with no notable change in Tm and LHF.9 In their solar chimney, Fadaei et al. employed PW as the PCM. The Tm and LHF of the paraffin utilized were recorded to be 44-46 °C and 189 kJ/kg, respectively. And it was discovered that using PW as PCM improved the solar chimney's thermal efficiency.10 Omara et al. studied the thermal performance of a solar water heating system combined with PCM that used a flat plate as a heat source in their study. The system's total energy and exergy were determined to be 85 and 76 %, respectively, and the recorded Tm of paraffin PCM was found to be 56 °C.11 Agarwal and Sarviya12 measured the Tm of commercial and LHF paraffin samples as 41-55 °C and 176 kJ/kg, respectively. Commercial PW was determined to be suitable for sun dryer applications based on the findings of the trial. Mahdi et al.13 conducted an experimental study on latent heat thermal helical coil thermal energy storage for melting and solidification phases. PW (type P56-58) was chosen for the job. PW was been determined to have Tm and LHF of 48.3-62 °C and 114.5 kJ/kg, respectively. The results demonstrated that coiled tube latent heat energy storage may be effectively utilized in solar thermal applications. To minimize the absolute drying time of crops in typical sun dryers, Vijayrakesh et al. used PW with Tm equal to 53.7 °C and LHF equal to 190 kJ/kg as PCM-packed floor.14 Bhagyalakshmi et al. developed a binary eutectic mixture of palmitic acid (60%) and PW (40%). The recorded Tm, solidification temperature, latent heat during charging, and latent heat during discharging were 52.6–59.8 °C, 40.9–47.1 °C, 214.7 kJ/kg, and 194.6 kJ/kg respectively.15
Thermal energy storage (TES) systems still confront some serious challenges including the stability and reliability of PCM/construction material composites. The PCM must remain useful for an extended length of time in the thermal system. Another major concern is that after repeated cycles of heating and cooling, long-term use of PCM leads to leakage from the building material. Another issue is the thermal stress caused by PCMs and corrosion, which leads to the downfall of the building material.5,16,17 In addition, PCMs for low-temperature applications are scarce. A lot of studies have been carried out during the last few decades, particularly in this direction, however, there is still a need for commercial PCM especially working between 30-60 °C, and should be widely accessible and reasonably priced in the local market.
A eutectic mixture is a mixture of multiple solids that melts or solidifies at a single temperature lower than the constituents' melting points. Generally, the melting point is sharp, and it has a somewhat larger storage density than the parent materials. The authors in their study chose capric acid (CA) and PW because of their high LHF, appropriate heat transfer properties throughout the phase change transition, easy availability, and low cost of the material. Aside from that, there is a scarcity of PCMs operating in the temperature range of 25-32 °C for construction and solar applications, and those that are available are considerably more expensive than other PCMs. The study's overall purpose is to create low-cost PCMs for TES applications. Binary mixes based on 98.5 % purity CA and laboratory grade (LR-grade) PW with varying weight ratios (10/90, 20/80, 30/70, 40/60, 50/50, 60/40, 70/30, 80/20,90/10, 92/08, 94/06, 96/04, and 98/02) were created for this purpose. The differential scanning calorimetry (DSC) method was used to assess their thermal properties. The developed PCM in the present study is cheap to buy (see Table 2) and readily accessible in India and abroad and can be employed for thermal energy applications.
The CA (purity 98.5%) and LR grade PW were procured from the Avra Synthesis Pvt. Ltd., Hyderabad, India, and SD fine-CHEM Ltd., Mumbai, India respectively, and the same was utilized without any purification for the preparation of mixtures. To determine the composition of the eutectic mixture, a sequence of binary combinations of CA and PW with different weight proportions were produced (10/90, 20/80, 30/70, 40/60, 50/50, 60/40, 70/30, 80/20,90/10, 92/08, 94/06, 96/04, and 98/02) from their liquid mixtures at constant temperature (70 °C), using magnetic stirrer with a hot plate. Thirteen such units (100g each) were made employing a mixture of CA and PW. These mixtures were initially in liquid form and then kept at room temperature for about an hour for gentle cooling and solidification. Throughout the experiment, an extremely precise and dependable semi-analytical digital balance with an accuracy of about ±0.0001 g was used to complete all weights measurements. The key thermal characteristics required to establish any material as a PCM for TES systems are LHF, Tm, and Tf. The Tm, LHF, of CA, PW, and their eutectic mixes were determined using the DSC method. In DSC, a sample and a reference material is heated in separate aluminum pans at a constant rate of temperature rise. Differences in temperature between the two are proportional to the difference in heat flow between them, and the DSC curve serves as the record. PerkinElmer DSC 4000 equipment was used to test the thermal behavior of all produced eutectics. The prepared samples were investigated between 15 °C and 80 °C at a 2 °C min-1 heating rate. Throughout the experiment, nitrogen was used as a purge gas, and the constant flow rate was maintained at 20 ml min−1. The largest deviation in enthalpy and temperature measurements was ±2% and ±0.1°C respectively. Thermal properties such as Tm, LHF, Tf, and LHC of CA, and PW procured from Avra Synthesis Pvt. Ltd. and SD fine-CHEM Ltd. respectively were measured by DSC with 2 °C scanning heating/cooling rate at 0th cycle and information provided from the manufacturer is listed in Table 1.
| Material | Tm range (°C) | Tma (°C) | Tpa (°C) | LHFa (kJ/kg) | Tf (°C) | LHCa (kJ/kg) | Purity (%) | Costb (US$ kg-1) |
|---|---|---|---|---|---|---|---|---|
| Capric acid | 29-31 | 31.60 | 34.14 | 167.53 | 28.04 | 172.58 | 98.5 | 20.96 |
| Paraffin wax | 58-60 | 50.33 | 58.84 | 202.86 | 60.33 | 213.38 | LR grade | 14.44 |
a Measured through differential scanning calorimetry (DSC) with 2°C scanning heating/cooling rate at zeroth cycle.
b One US$ = 76.3065 INR (15 December 15, 2021, 14:10:00 UTC, https://www.google.com/finance/quote/USD-INR).
The CA’s DSC curve has a single, sharp peak that represents the solid-to-liquid transition (Figure 1). The PW’s DSC curve (Figure 2) contains two peaks: the first, which is not as sharp and significant suggests a solid-solid transition, and the second, which is sharp and significant, shows a solid-liquid transformation.12 Figures 1 and 2 show the melting curve of CA and PW respectively, measured using DSC with a 2 °C scanning heating and the cooling rate at 0th cycle.
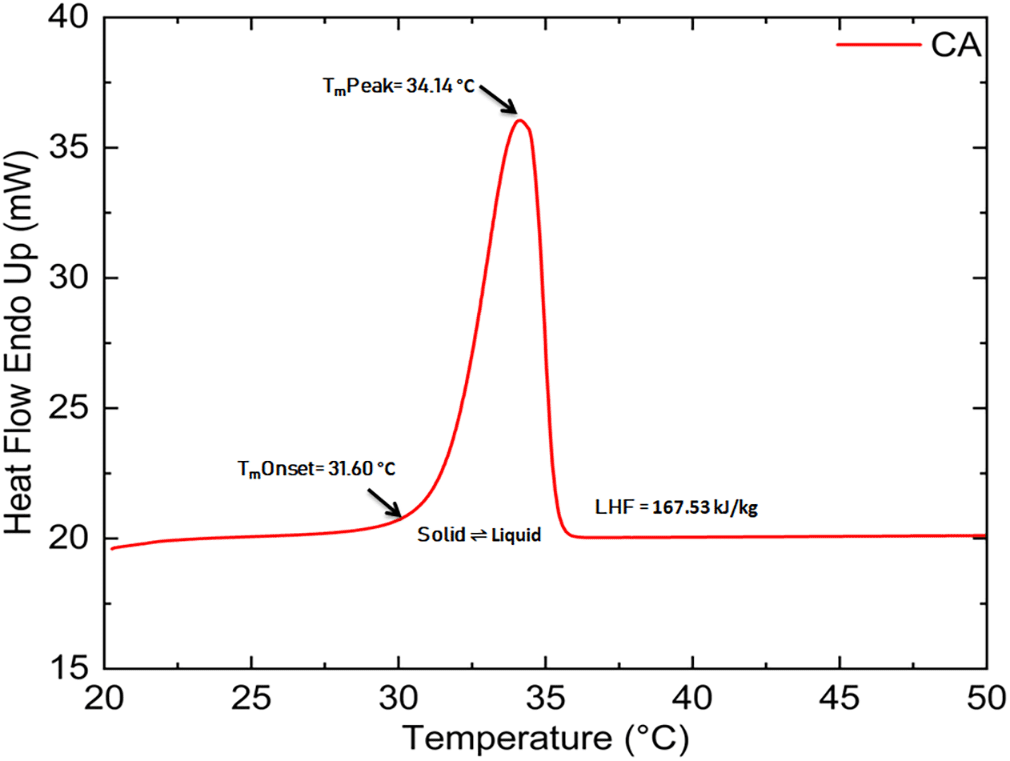
The onset melting point, peak melting point, and LHF of the prepared samples are listed in Table 2. Based on the result, it is clear that the melting point of mixes increases with decreasing the mass ratio of PW until it reaches 8%, and then it decreases at 6% and a further decrease in mass percentage again increases the melting point. In the case of LHF, it was discovered that raising the mass percentage of CA in the mixture causes LHF to rise. LHF of developed mixtures grew until the CA amount reached 94%, after which it began to decline. Figure 3 shows the DSC melting thermograph of a binary eutectic mixture of CA and PW (PWCA-0694). Most of the developed mixes have sufficient LHF value, and based on their melting point range (26.14 °C-30.60 °C), they can be used for TES applications in solar absorption chillers, buildings, surgical dress, clinical beds, and photovoltaic systems.5,16,17
| Sample code | Wt. % | First peak | Second peak | Cost (US$ kg-1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| PW | CA | Onset pointa (°C) | Melting peaka (°C) | Latent heata (kJ/kg) | Onset pointa (°C) | Melting peaka (°C) | Latent heata (kJ/kg) | ||
| PWCA-0298 | 2 | 98 | 30.60 | 33.70 | 139.94 | 20.82 | |||
| PWCA-0496 | 4 | 96 | 30.42 | 33.21 | 157.81 | 20.69 | |||
| PWCA-0694 | 6 | 94 | 30.16 | 33.31 | 198.62 | 20.56 | |||
| PWCA-0892 | 8 | 92 | 30.21 | 33.65 | 154.15 | 20.43 | |||
| PWCA-1090 | 10 | 90 | 29.86 | 32.83 | 162.22 | 20.30 | |||
| PWCA-2080 | 20 | 80 | 29.46 | 32.75 | 134.38 | 19.65 | |||
| PWCA-3070 | 30 | 70 | 29.17 | 32.89 | 120.06 | 19.00 | |||
| PWCA-4060 | 40 | 60 | 28.57 | 32.03 | 113.88 | 18.35 | |||
| PWCA-5050 | 50 | 50 | 28.32 | 31.48 | 103.86 | 17.70 | |||
| PWCA-6040 | 60 | 40 | 27.69 | 30.79 | 81.34 | 17.04 | |||
| PWCA-7030 | 70 | 30 | 27.35 | 29.67 | 46.07 | 16.39 | |||
| PWCA-8020 | 80 | 20 | 26.89 | 28.98 | 41.20 | 40.49 | 55.40 | 149.11 | 15.74 |
| PWCA-9010 | 90 | 10 | 26.14 | 28.14 | 15.05 | 46.56 | 57.84 | 154.33 | 15.09 |
To check the effect of different heating and cooling rates on Tm, different heating and cooling rates were used for the 400th thermal cycle of the CA/PW eutectic mixture to examine the influence of scan rate on Tm and LHF. The Figure 4 displays the CA/PW eutectic mixture (94/06 wt.%) DSC curve for the 400th heating and cooling cycle at 1 °C min-1, 2 °C min-1, and 5 °C min-1 scan rates. To investigate whether the heating/cooling pace has any influence on Tm, CA/PW, eutectic mixture (94/06 wt.%) in a cyclic manner was scanned at different heating rates (1, 2, and 5 °C min-1) under a constant flow of nitrogen at a flow rate of 20 ml min-1. During solid-liquid transition different scan rates produced different heat flow magnitudes. The DSC curve of the CA/PW eutectic mixture (94/06 wt.%) for various heating and cooling rates exhibited a smaller peak for low scan rate and a bigger peak for high scan rate Figure 4. The onset melting point did not change much with different scan rates because the leading edge slope remained constant. Based on the findings, it is reasonable to say that the onset melting point is independent of heating and cooling rates. Figure 4 shows, with increasing scan rate, the peak melting point shifts towards higher temperature. LHF for different scan rates can be approximated by peak area divided by scan rate.18 From the Table 3, it can be seen that for different scan rates LHF is nearly the same. And the table depicts Tm and LHF of PWCA-0694 for the 400th heating/cooling cycle for different scanning rates. Based on the findings, for the 400th heating/cooling cycle, there is not much difference in Tm and LHF at different scan rates, indicating the thermal behavior of samples to be similar at different heating and cooling rates.
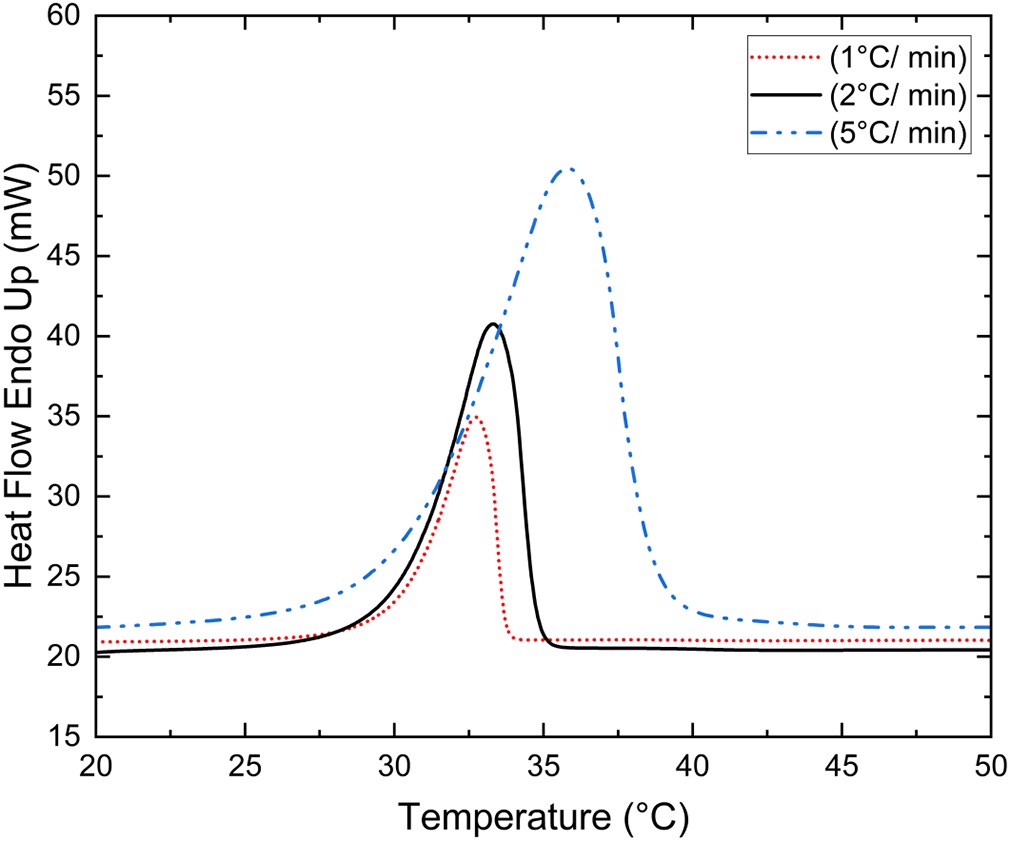
| Scan Rate [°C min-1] | Onset pointa [°C] | Melting peaka [°C] | Latent heat of fusiona [kJ/kg] |
|---|---|---|---|
| 1 | 30.25 | 32.74 | 162.18 |
| 2 | 30.20 | 33.58 | 158.98 |
| 5 | 30.21 | 35.83 | 156.30 |
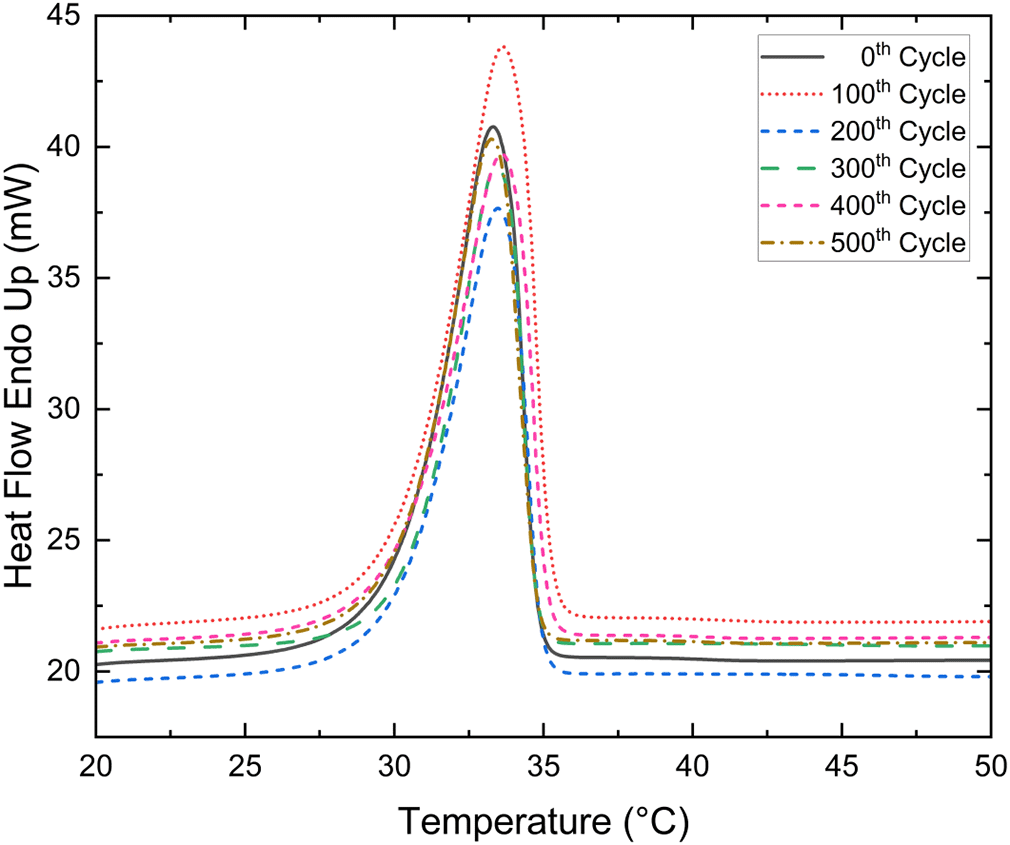
The Tm and LHF of a binary eutectic mixture (PWCA-0694) were measured after every 100th heat cycle, for a total of 500 cycles. The Figure 5 shows the DSC curves for the 0th- 500th thermal cycle at the succession of 100 thermal cycles of eutectic PCM. The maximum deviation in Tm and LHF was found to be +0.36 °C and -39.65 kJ/kg respectively as shown in Figure 6. The negative and positive values of Tm and LHF are solely relative to the 0th cycle, where positive represents an increase in value and negative represents a decrease in value relative to the 0th cycle. The Tm and LHF variation of the developed eutectic mixture is within acceptable limits. The developed mixture was thermally stable up to 500 cycles.
The PCMs developed by the authors' group are fairly less expensive in contrast to materials already in use in the market for alike applications (see Table 2). Whether, as per the information received from the local vendors, the cost of our CA is 20.96 $/kg and PW is 14.44 $/kg in the Indian market. The cost of the developed eutectic mixture is 15.09 – 20.82 $/kg, which may be further reduced by 30% to 40% if mass production is done.
The PCM development for TES applications is presented in this paper, which is based on binary mixes of CA and PW with various weightratios. The DSC technique was used to analyze the thermal characteristics of these binary blends, which revealed that a few of the developed materials had suitable phase change temperatures and high LHF. The most promising mass percentages of PW contained in CA were determined to be 02–10 wt.%, with reduced Tm of 29.86 °C - 30.42 °C and LHF 154.15–198.62 kJ/kg. From DSC analysis, it was found that the Tm of mixes raises with decreasing the mass percentage of PW until it reaches 8%, and then it decreases to 6% and a further decrease in mass percentage again increases the melting point. In the case of LHF, it was discovered that raising the mass percentage of CA in the combination causes LHF to rise. LHF of developed mixtures grew until the CA percentage reached 94%, after which it began to decline. The developed mixes were found to be suitable for TES applications in buildings, solar absorption chillers, surgical dress/clinical beds, and photovoltaic systems. Because of congruent mixing, desired melting range, and high LHF, PWCA0694 (CA 94 wt.% and PW 06 wt.%) was found to be the most promising mixture of all. From DSC analysis, its melting point was found to be 30.16 °C and LHF to be 198.62 kJ/kg and can be used for application in buildings and photovoltaic systems. Per the findings, different heating and cooling pace have virtually negligible influence on PWCA0694's Tm and LHF. It was also found that with increasing scan rate, the peak melting point shifts towards higher temperature. DSC analysis of 400th thermal cycles with varied scan rates revealed almost identical results, demonstrating that the thermal characteristics of the sample were constant except for the peak melting point, which rose with increasing heating rate. Accelerated thermal cycling test confirmed the structural and thermal stability of our developed PCM up to 500 thermal cycles. From the investigation, it was also found that the maximum percentage difference in Tm and LHF of PWCA0694 was +0.36°C and -39.65 kJ/kg respectively, which were within an acceptable range. The cost of our developed eutectic is cheap and are easily available in Indian or abroad market. If mass production is done then it can further reduce by 30-40%.
Figshare: DSC data, https://doi.org/10.6084/m9.figshare.21424239.v2. 19
Figshare: Cost analysis data, https://doi.org/10.6084/m9.figshare.21424491.v1. 20
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Thermal management materials
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Thermal Energy Storage and Phase Change Materials
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 Nov 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)