Keywords
Barracuda fish, Heavy metals, Histamine, Polycyclic aromatic hydrocarbons, Smoked fish
This article is included in the Agriculture, Food and Nutrition gateway.
Barracuda fish, Heavy metals, Histamine, Polycyclic aromatic hydrocarbons, Smoked fish
Barracuda fish can be processed into a smoked fish product. Barracuda fish production in Indonesia in 2017 was 22,118 tons.1 Despite their superior nutritional content, barracuda fish are prone to deterioration of quality, so adequate processing is needed. One kind of processing method is smoking. The smoking technique represents a series of chemical, thermal, and biochemical processes that occur in salted products. Currently, this technology is applied in various forms to process 40-60% of total meat products. Smoking is defined as the penetration of volatile materials resulting from the thermal damage of wood into the surface of fish.2 Smoking improves organoleptic characteristics, causes water loss, and prevents the growth of food microbes due to heat, aromatic substances, and bactericides from the smoke.3 The smoking method most commonly applied in Indonesia is traditional smoking.
The smoking process uses high temperatures, and the product is often dried first at a temperature of 40-50oC for 30 minutes to dry the surface of the product, followed by a smoking stage with a high temperature reaching 80-100oC.4 The traditional smoking process implemented commercially in Indonesia still uses simple equipment, and sanitation is typically not monitored; thus, sanitation efforts to prevent contamination during the smoking process require attention.
Traditional smoking processes with high temperatures can cause contamination of the resulting products, such as chemical and bacterial. The danger of chemical contamination includes heavy metal and harmful polycyclic aromatic hydrocarbons (PAHs) contamination. The increase in heavy metal content in smoked fish products is due to contamination during the combustion of fuel in the smoking process;5 for example, the content of Pb in goldfish, rainbow trout, and northern pike was shown to increase after smoking when smoked fish and fresh fish were compared.6 The danger of contamination with pathogenic bacteria such as Escherichia coli, Staphylococcus aureus, Salmonella, and Bacillus aureus has also been detected in smoked fish products.7 Liquid smoke can be used as an alternative to replace the traditional smoking.
Liquid smoke is a solution of compounds that are evaporated simultaneously during pyrolysis and condensed in cooling system. The chemical composition of liquid smoke depends on the type of wood used, the moisture content of the wood, the pyrolysis temperature, and the duration of smoke formation. Commercial liquid smoke has been shown to be effective against various types of putrefaction by pathogenic microorganisms.8,9
A large amount of research on the application of liquid smoke in food has been reported. The application of liquid smoke from coconut shells at a concentration of 5% was shown to extend the durability of smoked skipjack tuna during storage for 4 days at room temperature.10 This study aimed to evaluate the nutrition, chemical and total bacteria of smoked fish processed using traditional and liquid smoke methods.
10 kg fresh barracuda fish (Sphyraena sp.) were procured deceased. All fish samples were purchased from the Fish Auction Hall at Fish Landing Area, located in Jepara, Central Java, Indonesia. The fish were then put into a Styrofoam box containing ice in fresh condition and were brought to the Fish Processing Laboratory, Universitas Diponegoro, Indonesia for smoking by the two different methods, traditional and liquid smoke.
Fresh barracuda fish were cleaned and washed using clean water. Then, the fish were gutted. All of the fish that were gutted were then washed and drained. The traditional smoking method used a smoking furnace for approximately ±15 minutes. The liquid smoke method was carried out by immersing the fish in a 5% liquid smoke for 30 minutes, drained for 30 minutes, then heated gradually, at a temperature of 40-45°C for 1 hour; 60-70°C for 1 hour; and 90°C for 1 hour.11
The process of making liquid smoke from coconut shell is carried out using a series of liquid smoke production equipment consisting of a pyrolysis reactor, heating tank, condenser, liquid smoke reservoir, and cooling system.
The proximate analysis of moisture, ash, and fat content was carried out according to the AOAC method.12
The moisture content analysis begins with drying porcelain dish in an oven at 105oC for 1 hour. The weighing porcelain dish is then placed in a desiccator and allowed to cool and then weighed. Sample weighing 3-4 g were weighed. The weighing porcelain dish with sample was put in an oven at a 105oC for 5-6 hours. The weighing porcelain dish was put into a desiccator and allowed to cool then weighed and repeated the procedure until a constant weight was obtained. The percentage of moisture content (wet basis) can be calculated by the formula:
A: Weight of empty cup (g)
B: Weight of cup filled with sample (g)
C: Weight of cup with dried sample (g)
Analysis of ash content begins with drying the ashing dish in the oven for 1 hour at 105oC, then weighing it. A total of 5 grams of the sample was put into an ashing dish and ignited over a Bunsen flame until it no longer smoked, then put into an ashing furnace at 600oC until complete ash was obtained. After that, it was weighed until a constant weight was obtained. Ash content can be calculated by the formula:
A: Weight of empty cup (g)
B: Weight of cup filled with sample (g)
C: Weight of cup with the sample has ash (g)
Fat content analysis begins with weighing 5 g of the sample (W1) and putting it into filter paper, then the wrapped sample is put into a fat flask that has been weighed with a fixed weight (W2) and connected to a Soxhlet tube. The fat sleeve was put into the Soxhlet tube extractor and rinsed with fat solvent. The extraction tube was mounted on a Soxhlet distillation apparatus and then heated at 40oC using an electric heater for 16 hours. The fat solvent in the fat flask is distilled until all the fat solvent has evaporated. When the distillation solvent is accommodated in the extractor chamber, then the solvent is removed and the fat flask is dried in an oven at 105oC, after which the flask is cooled in a desiccator to a constant weight (W3). Fat content can be calculated by the formula:
Standard calibration curves for the determination of cadmium (Cd) and lead (Pb) were obtained by measuring the absorption of the standard solution of each element at the optimum condition of the element. The range of standard solutions of Pb is 0.1-2.5 mg/L, while Pb and Cd are prepared by varying their concentrations in the range of 0.01-1.5 mg/L. The calibration curve is obtained by making a curve between the concentration and absorption of each element.13
Analysis of heavy metals (Pb, Cd, Hg, Sn, As) was performed following the methods reported by National Standardization Agency for Indonesia (SNI 2354.5:2011).14 Sample 5 g was put in a closed container, then 5 mL of concentrated HNO3 and 5 mL of concentrated H2SO4 was closed and left for 24 hours. The solution is then heated at 60oC for 30 minutes, then heating was continued at 120-150oC until a black precipitate was formed. After its cooled, 10 mL of 10% nitric acid was added and shaken until the black precipitate dissolved. In the next step, 3 mL of H2O2 was added and shaken, then the solution heated again for about 15 minutes. The solution resulting from digestion after cooling was filtered through Whatmann filter paper No.42, and put in a 50 mL volumetric flask and diluted to the mark. After that it was put into a 200 mL beaker, then analyzed for lead (Pb), cadmium (Cd), tin (Sn), and mercury (Hg) measured by atomic absorption spectroscopy at 283,3 nm for lead (Pb), and 228.8 nm for cadmium (Cd).
Histamine analysis began with making a p-phenyl diazonium sulfate reagent, by mixing 1.5 ml of cold 0.9% (w/v) sulfamic acid in concentrated HCl and 1.5 ml of 5% NaNO2, in a 50 ml standard flask. The mixture was kept in an ice bath for 5 minutes. The reagents stored in the ice bath were used 15 minutes after dilution with water and were stable for 12 hours. Next, the extraction step was carried out by weighing 5 g of fresh and smoked barracuda fish samples that were then homogenized with 20 ml of HCl solution and centrifuged at 3100 rpm for 10 minutes. The samples were then analysed spectrophotometrically. 5 ml of Na2CO3 was put into a test tube, and 2 ml of reagent was added slowly, mixed, and then added to a tube containing 1 ml of the residual solution collected in the extraction process. The absorbance of the resulting colour was measured immediately after 5 minutes using a UV-Vis spectrophotometer at a wavelength of 496 nm using distilled water as a blank. The histamine concentration in the sample was obtained from a standard curve according to the absorbance at 496 nm by regression analysis. The histamine concentration in the sample was calculated with the following formula:15
A is the histamine value obtained in μg/ml from the standard curve.
Analysis of benzo[a]pyrene was performed following the methods reported by Ref. 16, 10 g of sample plus 10 g of anhydrous sodium sulfate was homogenized with 100 mL of n-heptane-ether, homogenized and centrifuged again. The second supernatant was mixed with the first supernatant and then put in an alumina column. The first 50 mL of eluent was discarded and then 50 mL of the second eluent was collected and calibrated on a spectrophotometer at an excitation wavelength of 295 nm and emission of 403 nm. Benzo[a]pyrene standard is used with a concentration of 0-10 mg/ml for calibration.
Microbiological analysis of fresh barracuda and smoked barracuda included determination of total plate count (TPC), Escherichia coli, Salmonella sp., and moulds.
Total plate count (TPC) determination was carried out on fresh and smoked barracuda fish samples using the Petri dish count method based on Indonesian National Standard number 01-2332.3-2006.17 The 25 g sample was added to 225 mL of Butterfield’s phosphate-buffered solution and homogenized for 2 minutes. This homogenate is a 10-1 dilution solution. Then, using a sterile pipette, 1 mL of the homogenate was taken and put into 9 mL of Butterfield’s phosphate-buffered solution to obtain a dilution of 10-2. A further dilution (10-3) is prepared by taking 1 mL of the sample from 10-2 dilution into 9 mL of Butterfield’s phosphate-buffered solution. Each dilution was shaken at least 25 times. Then the same thing was done for the dilution 10-4, 10-5, etc.
Analysis of Escherichia coli using the most probable number (MPN) method was based on Indonesian National Standard SNI 01-2332.1-2015.18 A sample of 25 g was homogenized with 225 mL of 0.1% buffer phosphate water (1:9) using a stomacher, then serially diluted 101-103. Samples were transferred by pipette 1 mL each from each dilution into 3 series of Lauryl Sulfate Tryptose Broth (LSTB) tubes containing Durham tubes. Samples were incubated at 35oC for 24-48 hours. The gas bubbles that appear are observed in the Durham tube. The test result is positive if there are bubbles. The estimation test was not repeated. In the confirmatory test, positive cultures were transferred from the assay with an Ose needle to an Echerichia coli Broth (ECB) tube, then incubated at 37oC for 24-48 hours. The gas formed was observed as a positive result. The MPN table was used to determine the positive MPN result of ECB as the number of Escherichia coli per g/per ml. In isolation and identification, samples from positive Escherichia coli Broth tubes were straked on Eosin Methylene Blue Agar (EMBA), incubation at 37oC for 24 hours. Escherichia coli colonies with a diameter of 2-3 mm were black/dark in the center of the colony with or without a shiny metallic green color on EMBA.
Determination of Salmonella sp. was based on the method of Indonesian National Standard number 01-2332.2-2006.19 50 g of samples were put into a sterile container and 450 mL of Lactose Broth solution was added. The solution mixture was homogenized for 2 minutes, then the solution was transferred to a sterile container and left at room temperature for 60 minutes in a closed container. The solution was shaken gently and if necessary determine to pH 6.8, then incubated for 24 h ± 2 h at 35oC ± 1oC. The next step is enrichment. 0.1 mL of the incubated solution was transferred to 10 mL of Rappaport-Vassiliadis (RV) medium and 1 mL of the incubated solution was transferred to 10 mL of Tetrahionate Broth (TTB). The RV medium was incubated for 24 h at 42oC ± 0.2oC. Then it was shaken using vortex and the TTB streak was incubated in Hectoen Enteric (HE) Agar, Xylose Lysine Desoxycholate (XLD) Agar, and Bismuth sulfite Agar (BSA). Scratch into the same medium from a Broth RV or Selenite Cystine Broth (SCB). BSA, HE, and XLD plates were incubated for 24 h at 35oC ± 1oC. Then, the possible presence of Salmonella colonies was observed.
Analysis of total mould colonies was performed according to Ref. 20 with slight modifications. All equipment was sterilized using an autoclave at 121oC and a pressure of 15 psi for 15 minutes. Potato dextrose agar (PDA) (approximately 3.9 g) was added to 100 ml of distilled water, and then brought to a boil. The mixture was sterilized in an autoclave at 121oC and a pressure 15 psi for 15 minutes. Test tubes were prepared and coded I to III. Each test tube was filled with 9 ml of 0.9% NaCl, and sterilized. In the next step, 10 g of sample was crushed and then put into a 250 ml Erlenmeyer flask containing 90 ml of sterile 0.9% NaCl solution to achieve a 10 fold dilution. A total of 1 ml of the solution was taken and transferred to test tube I with a pipette to obtain a (100-fold dilution). From the test tube, 1 ml of the solution was pipetted again and transferred to the second tube as 1000-fold dilution. From each dilution, 1 ml of the solution was taken aseptically and transferred into sterile Petri dishes, homogenized, and allowed to freeze. The Petri dish was then arranged upside down in an incubator at 25-30oC and incubated for 24-48 hours. The number of mould colonies growing on the agar medium in Petri dish was counted. The counted colonies amounted to 30-300 colonies. The total number of mould colonies counted was then multiplied by dilution factor.
The moisture, total fat content, and histamine of fresh barracuda and smoked barracuda using different smoking methods are shown in Table 1, the heavy metals content are showed in Table 2, and the vitamins are showed in Table 3. The fresh barracuda fish’s moisture, total fat, and ash content were 74.27%, 0.54%, and 1.30%, respectively. The traditional smoked barracuda fish’s moisture, total fat, and ash content were 61.69%, 5.24%, and 3.68%, while smoked barracuda fish using liquid smoke were 64.46%, 5.63% and 1.04%, respectively. Based on the results of variance analysis, it was found that the traditional and liquid smoke methods had a significant effect (p < 0.05) on proximate composition (Tables 4-9).
| Metal content (mg/kg) | Treatments | Indonesian smoked fish standard (mg/kg) | ||
|---|---|---|---|---|
| Fresh barracuda fish | Traditional smoking | Liquid smoke | ||
| Pb | ND | ND | ND | Max 0.3 |
| Max 0.4** | ||||
| Cd | ND | 0.13 ± 0.007 | ND | Max 0.1 |
| Max 0.5** | ||||
| Hg | 0.14 ± 0.011b | 0.08 ± 0.404a | 0.11 ± 0.006ab | Max 0.5 |
| Max 1.0** | ||||
| As | 3.88 ± 1.681b | 1.93 ± 1.275ab | 0.73 ± 0.338a | Max 1.0 |
| Sn | ND | ND | 0.04 ± 0.000 | Max 40.0 |
| Dependent variable: Moisture | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected Model | 262.063a | 2 | 131.032 | 83.708 | .000 |
| Intercept | 40169.513 | 1 | 40169.513 | 2.566E4 | .000 |
| Treatment | 262.063 | 2 | 131.032 | 83.708 | .000 |
| Error | 9.392 | 6 | 1.565 | ||
| Total | 40440.968 | 9 | |||
| Corrected Total | 271.455 | 8 | |||
| Duncan | ||||
|---|---|---|---|---|
| Treatment | N | Subset | ||
| 1 | 2 | 3 | ||
| Traditional_smoked_barracuda | 3 | 61.6933 | ||
| Liquid_smoke_smoked_barracuda | 3 | 64.4600 | ||
| Fresh_barracuda | 3 | 74.2700 | ||
| Sig. | 1.000 | 1.000 | 1.000 | |
| Dependent variable: Total_fat | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected model | 48.179a | 2 | 24.089 | 15.082 | .005 |
| Intercept | 130.264 | 1 | 130.264 | 81.557 | .000 |
| Treatment | 48.179 | 2 | 24.089 | 15.082 | .005 |
| Error | 9.583 | 6 | 1.597 | ||
| Total | 188.026 | 9 | |||
| Corrected total | 57.762 | 8 | |||
| Duncan | |||
|---|---|---|---|
| Treatment | N | Subset | |
| 1 | 2 | ||
| Fresh_barracuda | 3 | .5400 | |
| Traditional_smoked_barracuda | 3 | 5.2433 | |
| Liquid_smoke_smoked_barracuda | 3 | 5.6300 | |
| Sig. | 1.000 | .721 | |
| Dependent variable: Ash | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected Model | 12.654a | 2 | 6.327 | 5.782 | .040 |
| Intercept | 36.361 | 1 | 36.361 | 33.227 | .001 |
| Treatment | 12.654 | 2 | 6.327 | 5.782 | .040 |
| Error | 6.566 | 6 | 1.094 | ||
| Total | 55.581 | 9 | |||
| Corrected Total | 19.220 | 8 | |||
| Duncan | |||
|---|---|---|---|
| Treatment | N | Subset | |
| 1 | 2 | ||
| Liquid_smoke_smoked_barracuda | 3 | 1.0433 | |
| Fresh_barracuda | 3 | 1.3067 | |
| Traditional_smoked_barracuda | 3 | 3.6800 | |
| Sig. | .768 | 1.000 | |
The heavy metals contents of fresh and smoked barracuda fish are shown in Table 2. The arsenic (As) of fresh barracuda and traditional smoked barracuda is higher than the liquid smoke method, at 3.88 mg/kg, 1.93 mg/kg, and 0.73 mg/kg, respectively. The mercury (Hg) of fresh barracuda fish, traditional smoked fish, and smoked barracuda fish using liquid smoke were 0.14 mg/kg, 0.08 mg/kg, and 0.11 mg/kg, respectively. The cadmium (Cd) in traditionally smoked barracuda was 0.13 mg/kg, while fresh barracuda fish and smoked fish processed using liquid smoke did not contain any cadmium (Cd). A small amount of Tin (Sn) was reported in liquid smoked fish (Table 2). Based on the results of variance analysis, it was found that two smoking methods had a significant (p < 0.05) effect on the levels of mercury (Hg) and arsenic (As) (Tables 10-13). The histamine content is shown in Figure 1; in the traditional method the level was 67.63 mg/kg, while in fresh barracuda fish and liquid smoke method, no histamine was reported.
| Dependent variable: Heavy_metal_As | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected Model | 15.160a | 2 | 7.580 | 4.979 | .053 |
| Intercept | 42.815 | 1 | 42.815 | 28.122 | .002 |
| Treatment | 15.160 | 2 | 7.580 | 4.979 | .053 |
| Error | 9.135 | 6 | 1.522 | ||
| Total | 67.110 | 9 | |||
| Corrected Total | 24.295 | 8 | |||
| Duncan | |||
|---|---|---|---|
| Treatment | N | Subset | |
| 1 | 2 | ||
| Liquid_smoke_smoked_barracuda | 3 | .7300 | |
| Traditional_smoked_barracuda | 3 | 1.9333 | 1.9333 |
| Fresh_barracuda | 3 | 3.8800 | |
| Sig. | .277 | .102 | |
| Dependent variable: Heavy_metal_Hg | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected model | .006a | 2 | .003 | 5.130 | .050 |
| Intercept | .113 | 1 | .113 | 188.907 | .000 |
| Treatment | .006 | 2 | .003 | 5.130 | .050 |
| Error | .004 | 6 | .001 | ||
| Total | .123 | 9 | |||
| Corrected total | .010 | 8 | |||
| Duncan | |||
|---|---|---|---|
| Treatment | N | Subset | |
| 1 | 2 | ||
| Traditional_smoked_barracuda | 3 | .0833 | |
| Liquid_smoke_smoked_barracuda | 3 | .1067 | .1067 |
| Fresh_barracuda | 3 | .1467 | |
| Sig. | .288 | .092 | |

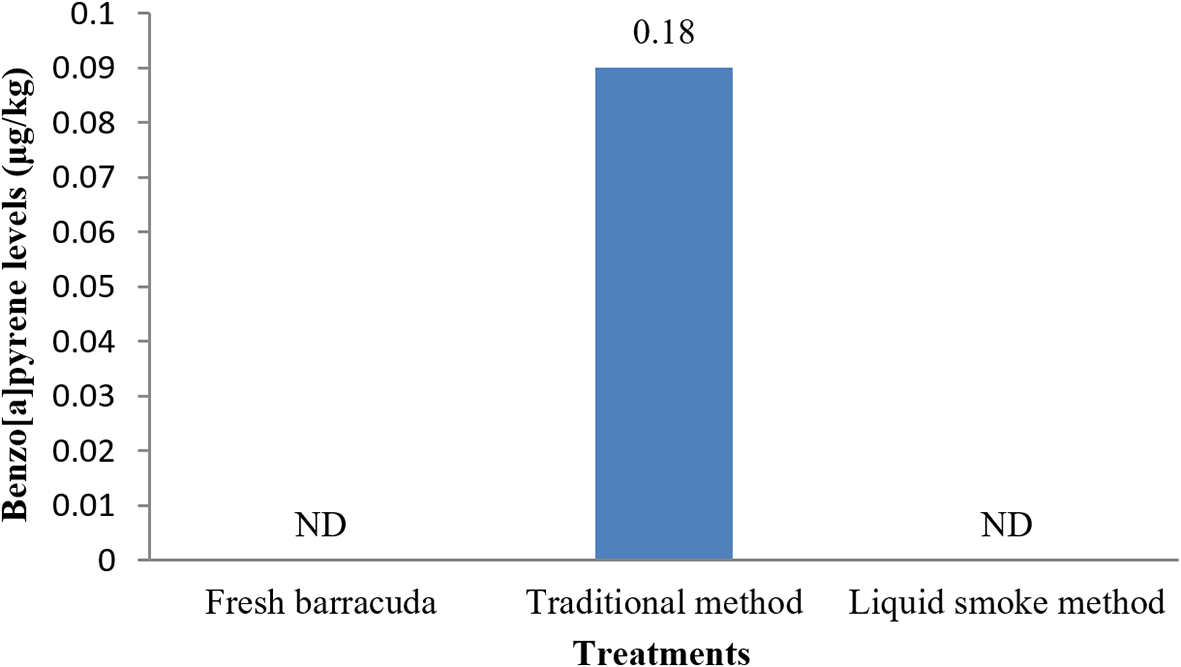
Barracuda fish processed using the traditional smoking method in this study showed a total benzo[a]pyrene content of 0.18 μg/kg, while fresh barracuda fish and barracuda fish treated with the liquid smoke method did not contain defectable levels of benzo[a]pyrene (Figure 2).
The total bacteria for fresh barracuda fish was 7.84 × 103 colonies/g, while those of barracuda fish smoked using the traditional method and the liquid smoke method were 4.2 × 102 colonies/g and 4.6 × 101 colonies/g, respectively. Other microbial contaminants, such as Escherichia coli and Staphylococcus aureus were observed at levels of < 3 APM/g and < 10 colonies/g, respectively (Table 3).
The quality of fresh and smoked fish can be seen from the proximate content, including moisture content, total fat content, and ash content. Moisture content is the most important parameter because it greatly effects the shelf life of a product and also affects the growth of microorganisms. The moisture content of fresh barracuda fish decreased after the smoking process (Table 1) because the moisture content in the food material evaporated and was absorbed by the air in the traditional stoves and ovens. Smoked barracuda fish prepared with traditional and liquid smoke methods had a water content that still exceeded the maximum limit in the Indonesian National Standard for smoked fish, of 60%.21 However, liquid smoke has an antagonistic function, namely, it can kill bacteria at high moisture contents. The smoked fish has the lowest moisture content (9.6 ± 0.16%) of other smoking methods, such as the combination of sundried and smoke or the combination of salted and smoked methods.22 Tilapia fish chikuwa with liquid smoke addition has a lowest moisture content (63.54%).23 The moisture content of smoked fish processed using re-distilled liquid smoke ranges from 48.19% to 57.2%.24 The moisture content of barracuda fish decreases inversely proportional to the total fat content. The total fat content of barracuda fish increases along with the decrease of the moisture content because of heating in the smoking process.25
The total fat content of smoked barracuda fish in the traditional smoking method (5.24%) was lower than the total fat content in the fish smoked using liquid smoke (5.63%), which were not significantly different (p ≤ 0.05). Changes in fat levels in fish during processing can be caused by several factors such as processing, temperature, and reactions with chemical compounds in liquid smoke.23 Besides that, the distance between the heat source and fish in traditional stoves is very close; due to high smoking temperature, the fat in barracuda fish is damaged, and the total fat content decreases.26
Analysis of ash content needs to be performed because it is used to determine whether a food should be consumed. The ash content of the fish subjected to the traditional smoking method was higher than that of the sample subjected to the liquid smoke method (Table 1). The increase in ash content was inversely proportional to the decreasing moisture content. The ash content of smoked barracuda fish can be influenced by the salt used in the soaking process when preparing smoked fish. The changes in ash content were closely related to the NaCl content in the sample. The addition of NaCl increases the amount of sodium in a sample, resulting in an increase in ash content.27
The heavy metals lead (Pb), cadmium (Cd), mercury (Hg), arsenic (As), and tin (Sn) are dangerous and can cause poisoning in humans. Smoked barracuda fish prepared with either smoking method exhibited levels of cadmium (Cd) below the maximum limit of the Indonesian National Standard (Table 2), which is 0.5 mg/kg21, as well as relatively low levels of lead, mercury, and tin.
The level of the heavy metal arsenic in the fish subjected to the traditional smoking method exceeded the maximum Indonesian standard limit for smoked, while the liquid smoke method has met the Indonesian standard for smoked fish (maximum 1.0 μg/kg). Arsenic levels that exceed the maximum limit can cause toxicity in human body, and it can be harmful to the eyes, skin, blood, and liver. The effects of arsenic can interfere with vision and peripheral eye contractions, cause infection of the skin (dermatitis), cause bone marrow function failure and lead to a decrease in the number of peripheral blood cells. Prolonged exposure to arsenic can cause liver tissue to turn into connective tissue (liver cirrhosis), and can lead to the accumulation of fluid in the abdominal cavity (ascites). Arsenic exposure in the body can also cause kidney damage, especially renal damage (tissue damage).28
Histamine is biogenic amine compound that plays an important role in physiological functions, but if the amount of histamine is excessive, it can cause poisoning in the consumer.29 As shown in Figure 1, histamine was not detected in fresh barracuda fish. This could be because the histamine levels were very low in the fresh barracuda fish. Fresh fish do not contain free histamine but contain the amino acid L-histidine. Histamine is formed in fish by certain bacteria capable of producing the enzyme histidine decarboxylase, which can convert free histidine into histamine.30
The histamine levels in barracuda fish increased after smoking using the traditional smoking method, reaching 67.63 mg/kg. The increase in histamine content was caused by unhygienic handling during the smoking process and the growth of histamine-producing bacteria. Significantly high amounts of biogenic amines are produced during seafood processing as a result of microbial contamination through decarboxylation of specific free amino acids by exogenous decarboxylase enzymes released by seafood-associated microorganisms.31
In smoked barracuda fish products processed using the liquid smoke method, no histamine was detected. This was caused by phenolic compounds and acetic acid, which act as antibacterial agents. Liquid smoke can slow the growth of microbes by lowering the pH. Histamine-forming bacteria can be halotolerant or halophilic, and some bacteria are better able to produce histamine at low pH levels. As a result, it is possible for histamine formation to occur during processes such as smoking, drying, and fermentation.32
Based on the results of this study (Figure 1), it was found that the traditionally smoked barracuda fish product had a histamine content lower than the requirements of the Indonesian smoked fish standard (SNI 2725:2013), 100 mg/kg. This shows that smoked barracuda fish are safe for consumption. Consumption of foods that contain excess histamine can cause poisoning and disease. The human body can detoxify low histamine levels, but consuming foods containing histamine above 200 mg/kg can cause disease. After histamine is released into the bloodstream, various symptoms can be observed.33 The most common symptoms are tingling, rash, a drop in blood pressure, vomiting, headache, dizziness, nausea, diarrhea, heart palpitations, and respiratory distress.34 Various countries have set legal limits for the consumption of foods containing histamine such as 50 mg/kg35 and 100 mg/kg.36 At first glance the symptoms of histamine poisoning are similar to allergic symptoms that occur in general in people who have hypersensitivity to certain stimuli at doses tolerated by individuals.37
Benzo[a]pyrene has the highest carcinogenic value compared to others PAHs. Benzo[a]pyrene contributes anywhere from 1%-20% of the total carcinogenic effects found in smoked products.38
Barracuda fish processed using the traditional smoking method in this study showed a total benzo[a]pyrene content of 0.18 μg/kg, while fresh barracuda fish and barracuda fish treated with the liquid smoke method did not contain detectable levels of benzo[a]pyrene. The distance between fish and smoke sources in traditional smoking is also an important factor that must be considered. PAHs are bound to smoke particles. Longer distances between fish and the smoke source can reduce the number of PAHs in the fish.39 Benzo[a]pyrene content in coconut and paddy liquid smoke have been studied. Coconut shell and paddy liquid smoke did not have detectable levels, because the temperature of pyrolysis < 400oC was lowered in liquid smoke processing.40 Benzo[a]pyrene in smoked Nile tilapia using corn cob and coconut shells liquid smoke was not detected.41 The maximum acceptable level for benzo[a]pyrene is 5.0 μg/kg.21
Benzo[a]pyrene can be carcinogenic; this is due to the nature of benzo[a]pyrene which is hydrophobic (does not like water) and does not have a methyl group as other reactive groups to be converted into more polar compounds. As a result, PAH compounds are very difficult to excrete from the body and usually accumulate in the liver, kidney, adipose tissue, or body fat. With molecular structures similar to nucleic bases (adenosine, thymine, guanine, and cytosine), PAH molecules can easily insert themselves into DNA strands. As a result, DNA function will be disrupted and if this damage cannot be repaired in cells, it will cause cancer.42
The principle of the fish smoking process is to suppress the growth of spoilage bacteria to extend the shelf life of the product. The comparison of microbial contamination of fresh barracuda fish with smoked barracuda is presented in Table 3. Smoked barracuda fish processed using the liquid smoke method had the lowest total plate count, due to ability of liquid smoke ton inhibit the growth of microorganisms by lowering the pH. The decrease in pH value is caused by the metabolism of lactic acid bacteria.35
The smoked barracuda fish products processed using the traditional and liquid smoke methods met quality standards because they had total plate count values below the maximum limit for the requirements of the Indonesian smoked fish (SNI 2725:2013). The maximum total bacteria limit for smoked fish consumed is 5.0 × 104 colonies/g or a log value of 4.6921; thus, based on this value, smoked barracuda fish using the traditional method and the liquid smoke method are suitable for consumption.
Bacterial contamination of smoked barracuda can occur through air, soil, or improper handling of fish. An increase in moisture content during storage and an increase in the supporting temperature can also trigger bacterial growth, such as the growth of Staphylococcus aureus bacteria, which grow well at temperatures of 30-37°C.43 Liquid smoke fumigation is more hygienic than traditional smoking using a furnace so that it can reduce the number of Escherichia coli and Staphylococcus aureus bacteria. Coconut shell liquid smoke can act as an antibacterial on Pseudomonas aeruginosa and Staphylococcus aureus.44 Corncob liquid smoke is also reported to be able to inhibit the growth of Escherichia coli, Staphylococcus aureus, Vibrio harveyi, and Vibrio parahaemolyticus bacteria.45
Fresh barracuda fish and smoked barracuda fish (traditional method and liquid smoke method) obtained negative results for the pathogenic bacteria Salmonella sp., so the products were safe for consumption. The quality standard for Salmonella bacteria is based on Indonesian smoked fish (SNI 2725:2013).21 The antimicrobial activity of liquid smoke is caused by the presence of phenol and acid components.46 The mechanism of antimicrobial activity of phenols and their derivatives includes reactions with the cell membranes that can increase cell membrane permeability and result in the loss of cell contents, inactivation of essential enzymes and functional inactivation of genetic material.47
Mould testing was carried out to identify and count the number of mould colonies that resulted in a decrease in product quality. A high amount of mould will cause a decrease in the quality of smoked fish, promoting rancidity.48 The results of the mould test (Table 3) show that both fresh barracuda fish and smoked barracuda fish (traditional methods and liquid smoke methods) met the requirements of the Indonesian smoked fish.21
Liquid smoke is increasingly being used because it has a distinctive flavor and inhibitory effects on pathogens. The preservative effect of liquid smoke in these foods occurs because of the presence of antimicrobial and antioxidant compounds, such as aldehydes, carboxylic acids, and phenols.44 Therefore, liquid smoke has the potential to be used as a natural antimicrobial for food products. Liquid smoke also can make a product more flavoring.
Barracuda fish have good potential to be processed into smoked fish. Smoked barracuda fish products using liquid smoke can guarantee nutritional needs and product safety because the content of heavy metals, histamine, and benzo[a]pyrene met the levels set in the Indonesian National Standard. Very low total plate count values were detected in the smoked barracuda fish and were within the Indonesian National Standard limits.
Fronthea Swastawati: Conceptualization, Project Administration, Resources, Supervision, Validation, Writing-Review and Editing.
Retno Ayu Kurniasih: Conceptualization, Project Administration, Resources, Supervision, Validation, Writing-Review and Editing.
Putut Har Riyadi: Conceptualization, Resources, Supervision, Validation, Writing-Review and Editing.
Aninditya Artina Setiaputri: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Resources, Validation, Writing-Original Draft Preparation, Visualization.
Defita Faridlotus Sholihah: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Resources, Validation, Writing-Original Draft Preparation, Visualization.
figshare: Safety, quality, and nutritional aspect of smoked barracuda fish. https://doi.org/10.6084/m9.figshare.20201897.v149
This project contains the proximate analysis data.
figshare: Safety, quality, and nutritional aspect of smoked barracuda fish. https://doi.org/10.6084/m9.figshare.20201987.v150
This project contains the heavy metal analysis data.
figshare: Safety, quality, and nutritional aspect of smoked barracuda fish. https://doi.org/10.6084/m9.figshare.20202032.v151
This project contains the histamine and benzo[a]pyrene analysis data.
figshare: Safety, quality, and nutritional aspect of smoked barracuda fish. https://doi.org/10.6084/m9.figshare.20202011.v152
This project contains the microbiology analysis data.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Food technology, Food safety management
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: seafood science and technology; seafood nutrition; seafood preservation; amino acids, chromatography methods
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 24 Nov 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)