Keywords
browlift, minimally invasive, surgical technique, frontalis suspension, outpatient procedure, non-excisional browlift
browlift, minimally invasive, surgical technique, frontalis suspension, outpatient procedure, non-excisional browlift
Ptosis of the forehead or eyebrows is a common problem, especially among the elderly. Using the muscles that move the eyebrows, a lot of expressions can be communicated and can be easily recognized. Laterally inclining eyebrows transmit sadness, medially inclining eyebrows transmit anger, low eyebrows transmit tiredness, drawn up eyebrows transmit surprise and properly aligned eyebrows transmit an alert, rested state allowing the mouth to smile1,2. The position of the brow is affected by the corrugator supercilii, depressor supercilii, orbicularis oculi, and procerus (brow depressor muscles) and the frontalis muscle (brow elevator muscle)3. The motor innervation of these muscles is supplied by branches of the facial nerve. The temporal branch innervates the frontalis, superior part of the orbicularis oculi, the transverse head of the corrugator supercilli and superior part of the procerus muscles. The zygomatic branch innervates the inferior and medial parts of the orbicularis oculi, the inferior part of the procerus, the depressor supercilii and the oblique head of the corrugator supercilii muscles.
Common complaints of brow ptosis are a tired and heavy feeling of the eyes, problems watching television and reading, increased tearing and a limited field of vision1–6. Continuous activation of the scalp and forehead muscles may additionally cause tension headaches and eyestrain4. On closer examination, the cause of this may be partly due to a too low position of the eyebrows. As a result, the skin of the upper eyelids is pushed down, as it were, so that it looks like there is too much skin there. In patients with brow ptosis, unintended emotions can be shown, which can be misinterpreted by others5,6.
If the eyebrows are in a low position, a blepharoplasty alone makes little sense and sometimes no improvement occurs. It is better to first correct the cause of the deviation, the low position of the eyebrows, and then remove any remaining skin surplus from the upper eyelids. A wrong assumption is that the simultaneous treatment of blepharochalasis, and thus including a blepharoplasty procedure to the browlift procedure is not deemed beneficial. The added removal of upper eyelid skin in a single operation actually worsens the patient’s appearance due to excessive traction of the skin, causing an increase in their brow ptosis giving the patient a tired, older and angry appearance5,7,8. Therefore, patients desire a difference in brow stance that is both long-lasting and appears natural. Other factors that are included in decision-making are the visibility of scars, the cost of the procedure and practicality (procedure time, use of anaesthetics etc.).
Several techniques exist to treat ptosis of the eyebrows5,9–18. Some only treat brow ptosis, and other techniques are also used for treating a wide spectrum of facial aging changes. Direct browlift and the traditional fascial suspension technique tend to leave a very noticeable scar above the eyebrow15–18. Other options such as the (mid-) forehead browlift and coronal browlift can be used to elevate the brow in patients with deep creases who are not candidates for extensive surgical procedures, although significant scars are still made19.
Foreheadplasty, or open browlift, techniques have also been used to lift the eyebrows, namely the forehead (pretrichial) incision, corobregmatic incision, vertex incision, lambdoidal incision, W-incisions, lambdoidal paddle incision and the interlocking Ms5. These techniques take the same time as an endoscopic procedure and have the ability to adapt to various wrinkle, crease and forehead hairline considerations5. Other techniques, such as the transblepharoplasty or transpalpebral approach, use an upper blepharoplasty to resect the corrugator muscles and divide the procerus muscle along with temporal incisions to elevate the lateral eyebrow20,21.
In 1994 Vasconez et al.22 first described the endoscopic browlift technique, which has been widely studied10–12,14,23–32. This is a popular technique since advantages include less scarring, alopecia and numbness posterior to the scar. Despite a 70% satisfaction rate reported, the frequency of the endoscopic lift being performed by plastic surgeons has decreased33. The endoscopic browlift is also a costly and lengthy procedure that requires an operation theatre and can’t be performed on an outpatient basis. Additionally, reduced sensation has been described in endoscopic and open browlifts34.
Although many options exist in the treatment of brow ptosis, there is of yet no golden standard. The technique developed by Dr. L.T. Tan presented in our previous article35 combines traditional browlift approaches, such as the incision sites of the pretrichial and direct browlift without excising skin, with the dissection of frontalis muscle from the periost as used in the endoscopic browlift. Our technique found high satisfaction rates on scars (72.4%) and symptom improvement (72.4%) with minimal complications (long-lasting pain (n=4), cosmetic deterioration (n=3), noticeable subcutaneous lump (n=3), photophobia (n=1) and numbness (n=1)) and, in addition, does not require an operation theatre since only local anaesthesia is used. Thus our technique proves to be a more practical, cheaper alternative with optimal functional outcome and minimal scarring and therefore an optimal cosmetic result, especially when compared to more traditional browlift techniques. The aim of this study is to further evaluate the clinical and cosmetic data from patients who will undergo this procedure in the future and provide for more accurate measurement of the surgical effects.
The majority of the brown ptosis cases occur as weakening (e.g. involutional changes) or descent of the periorbital and/or facial soft tissue. This typically occurs at the temporal side of the brow first, mainly and the most temporal 1/3 of the eyebrow. Anatomically, the frontalis muscle raises the eyebrow and the frontal branch of the facial nerve is responsible for the innervation of this muscle. Normally the frontalis muscle lifts the medial 2/3 of the eyebrow and with increasing age, laxity of tissues (in particular collagen) combined with the descent of facial/periorbital soft tissue, patients develop lateral brow droop.
In terms of differential diagnosis, brow ptosis can occur with the following diseases: 1) paralysis or weakness of the frontalis muscle (facial nerve palsy, Bell’s palsy, acoustic neuroma, surgical trauma, birth trauma, congenital diseases1, myasthenia gravis, myotonic dystrophy, or oculopharyngeal muscular dystrophy); 2) involuntary contraction of the orbicularis oculi (blepharospasm or facial dystonias); and 3) mechanical causes, which can result in descent of the brow (neoplasms: basal cell carcinoma; squamous cell carcinoma; keratoacanthoma or melanoma).
There is a need to provide relevant evidence in patient care in (aesthetic) brow surgery, since most browlift techniques are done from experience and not necessarily evidence based. So therefore we took a number of key questions into account:
1. Will the browlift relieve complaints (increase worthwhileness)?
2. What is the longevity of the procedure?
3. How natural will the forehead region appear after surgery? This will be assessed via:
4. Will there be any unforeseen adverse effects? This will be assessed via numbness.
The primary objective of this study is to determine if our browlift technique is comparable or superior to existing types of browlifts using facial observation questionnaires administered pre- and postoperatively:
Research design. A prospective observational study will be performed on all available data from patients who undergo a browlift procedure in the Haaglanden Medical Center (HMC) from 1-6-2021 until 31-5-2022. The Medical Ethics board of the Haaglanden Medical Center judged that this research (protocol number N21.009/ML/ml) is mainly an evaluation of a common treatment method and therefore does not need a formal ethics approval procedure. Since this is an exploratory pilot study, no formal power and sample size calculation was done and we aim to include at least 50 patients (1 per week)
History
Every patient that presents with droopy eyelids and/or eyebrows should undergo a thorough medical and family history, including at least the following aspects:
Slowly progressive onset of the symptoms, together with a positive family history can indicate myotonic dystrophy or oculopharyngeal dystrophy.
Assessment of possible fluctuation of the symptoms or presence of fatigue (which can be present in case of myasthenia gravis).
A detailed history of surgery and/or trauma may point in the direction of damage to the frontal nerve or muscle scarring.
Symptoms that could indicate a facial palsy.
A detailed oncological history, stroke and/or head and neck tumours.
Physical examination
A complete plastic ophthalmic examination should be conducted in every patient, including:
- A visual acuity, pupillary and extraocular investigation
- A neurological examination of each cranial nerve
- The mentioned distances in figure 1 will be noted and measured
- A complete skin examination including if there is skin resting in the eye lashes
- The brow position will be noted in the situation of a relaxed frontalis muscle
- Possible prominent dynamic and static rhytids will be checked
- The location of the hairline will be noted
- In case of a paralytic brow, the physician will check for signs of facial trauma and/or scarring
All patients will be evaluated to check if there is dermatochalasis/ptosis and concomitant brow ptosis. Additionally, every patient scheduled for blepharoplasty will be checked if brow-repositioning surgery is necessary.
Pre-operatively the patient is informed about the procedure and information is given about (visible) scar formation, asymmetry and post-operative pain.
The patient is evaluated in the upright position. The degree of ptosis and position of the hairline is noted and compared with the contralateral side. Manual elevation of the brow to the ideal position will help to determine how much of the visual field deficit is secondary to the brow ptosis alone. Since exuberant brow lifting may compromise eye closure, attention should be paid to any degree of lagophtalmos if present. Secondly, injections with xylocaine + adrenalin 1:100.000 are administered into the dermis and subdermal until the periost of the lateral frontalis muscle region for adequate local anaesthesia. Two horizontal incisions (approximately 1-1.5 cm) are made, just above the lateral 1/3 of the eyebrow and the other just cranial to the hairline in line with the frontalis muscle. These locations are chosen to avoid injury to the frontal nerve (Figure 1). Secondly, the frontalis muscle is bluntly dissected from the underlying periost. The frontalis muscle is then suspended 2–5mm and fixated at the cranial site to the periost of the frontal bone using a 3-0 (Ethilon) suture. Then the muscle is also suspended 2–5mm at the caudal site using a 3-0 (firstly Ethilon, later Prolene) suture fixing it to the periost of the frontal bone. Attention is paid to achieve perfect symmetry between both sides. Finally, the skin is closed with Ethilon 5-0 sutures. The head is than wrapped with a bandage for 24–48 hours. The duration of the procedure is 20–30 minutes. For graphical depiction of technique, see Figure 2.
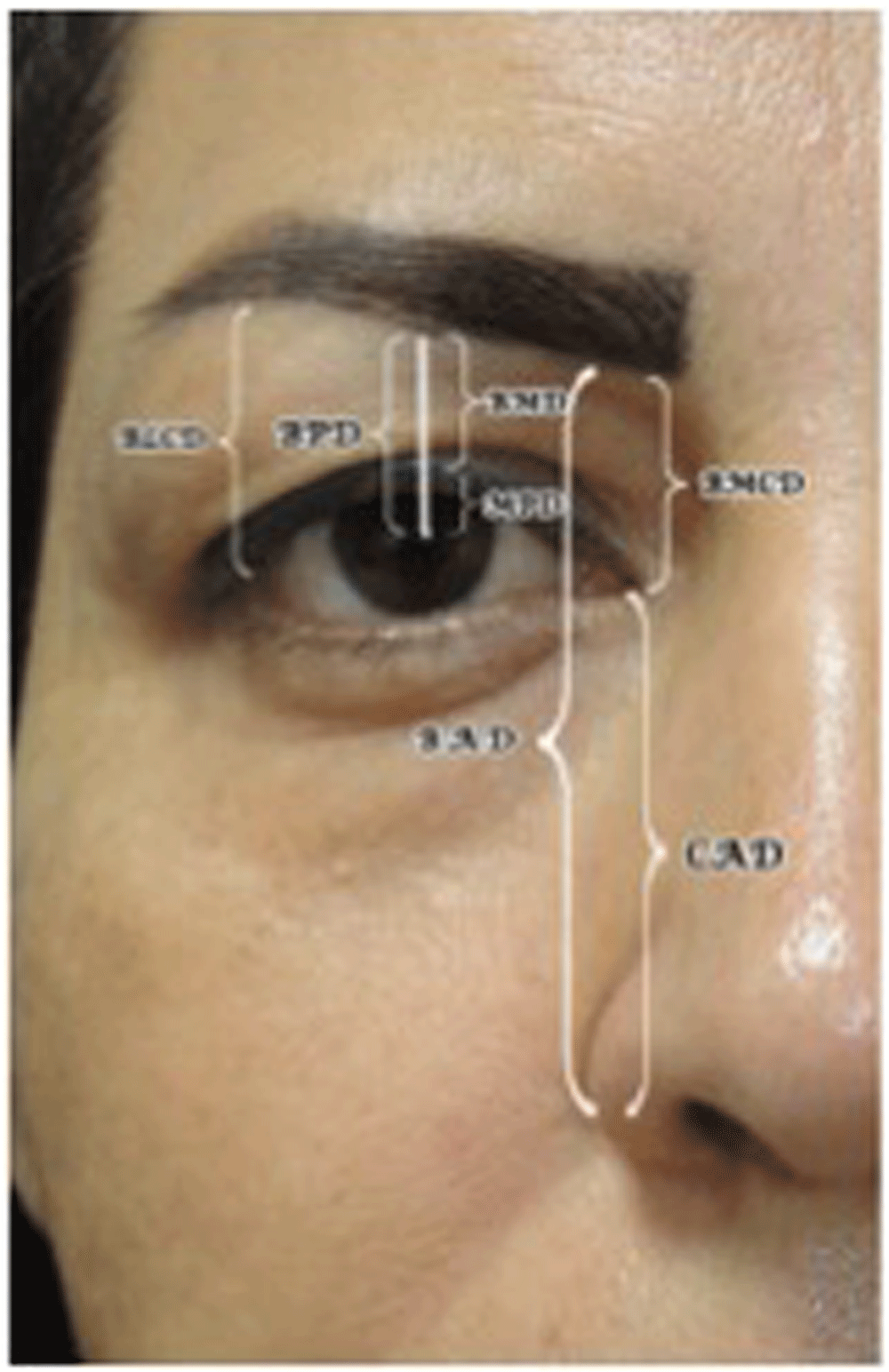
Measured with Emotrics software. *Informed consent was obtained for the usage of the patient’s photograph
BLCD= Brow-lateral canthal distance, BMD= Brow-lid margin distance, MPD= Lid margin-pupil distance, BPD= Brow-pupil distance, BMCD= Brow-medical canthal distance, CAD= Canthal-nasal alar distance, BAD= Brow alar distance, PF= palpebral fissure height. All distances to the pupil are measured to the centre of the pupil
Surgical complications are relatively uncommon. However, bleeding, numbness and tingling, injury to the facial nerve resulting in paralytic brow ptosis, infection and postoperative asymmetry have been described in previous browlift studies (25–35) and will be documented accordingly.
Patients with an indication for brow correction will receive information to review study specific information. Two weeks after patients have received the study information, they will be asked to participate in our study. see Table 1 for patient assessment moments.
Demographic information of patients will also be collected, such as age, race, and gender, as well as surgical indication and level of brow depression (see Figure 1).
Vectra XT. The Vectra XT 3D-imaging device (M3) produced by Canfield can be used for a wide variety of medical indications where accurate measurements can provide clear and clinically relevant information for both the practitioner and the patient.
A 3D-image can be easily made with the Vectra XT by correct positioning of the patient in front of the device. The device provides standard outlines, so correct positioning can be achieved relatively easily. The device and its accompanying software subsequently provide the practitioner with some standard measurements, which can be supplemented with additional measurements. (see Figure 1 for the overview). Additionally, the Vectra XT software can automatically provide before and after surgery differences in facial measurements by overlaying the produced photographs within one patient file.
Photographer instructions (Table 2)
- 3D imaging photographs can only be made at HMC Bronovo, where the Vectra XT is situated.
- Create a separate patient file for every patient, where the different assessment moments can be safely stored and lastly compared.
- Ensure correct positioning: align the patient’s eyes within the horizontal and vertical green bars which are provided within the standard Face Sculptor software.
- Inform the patient to sit still looking straight forward, ensuring that the patient looks at their own eyes in the centre of the mirror on the device.
- Once the 3D picture is made, measure the parameters as depicted in Figure 1 and save the photograph within the patient file clearly stating the assessment moment.
Patient instructions
To ensure high quality photos and for the best comparison it’s important that the patient has a neutral facial expression and is not wearing (or is wearing minimal) make-up.
- The patient should be seated to ensure stable positioning for optimal photography.
- To have a clear view of the patient’s face, their hair needs to be tied or tucked away such that none of the observed facial features are hidden.
For analyses we will use descriptive statistics and inferential statistics. A Kolmogorov-Smirnov test, a Q-Q plot and Levene’s test will first test all data for normality. Categorical variables will be expressed as n (%). Continuous normally distributed variables will be expressed by their mean and standard deviation, not normally distributed data by their median and interquartile range for skewed distributions. To test groups, categorical variables will be tested using the Pearson’s Chi-square test or Fisher’s exact test, when appropriate. Normally distributed continuous data will be tested with the independent samples Students t-test and in case of skewed data, with the independent samples Mann-Whitney U-test. Not normally distributed data will be tested by a Log rank test. When appropriate, for testing multiple possible factors for survival, a Cox proportional hazards analysis will be used. Statistical Package for Social Sciences (SPSS, Chicago, IL, USA Version 20.0) will be used to prepare the database and for statistical analysis.
The local data originating from the HMC-population will be coded. Each subsequent included and eligible case will be given a number, which is linked to the identifying patient details. The key to translate the code will be held by the main investigator in the HMC, Dr. de Jongh. The coded data will be stored by the aforementioned local investigators of this study in a spreadsheet that is secured by a password only known by these investigators. Only they will therefore have access to this data. Data will be kept in storage for 15 years. After the study ends the data will be made available on request. Each request will be assessed by dr. de Jongh.
This study will evaluate the quality of life related to and quantified data for this new browlift procedure. This will hopefully lead to an increased number performed under local anesthesia in an ambulatory setting. Follow-up studies should investigate our browlift technique in specific patient subgroups (for example patients with a peripheral facial palsy).
Ongoing; completion is expected in the first quarter of 2023.
The results of this study will be presented at scientific meetings and published in peer-reviewed medical journals.
figshare: Data Protocol Browlift Study. https://doi.org/10.6084/m9.figshare.17207579.v136
This project contains the following files:
- FACE-Q Age Appearance Appraisal.pdf
- FACE-Q Age Appraisal VAS.pdf
- FACE-Q Decision.pdf
- FACE-Q Face Overall.pdf
- FACE-Q Forehead and Eyebrows.pdf
- FACE-Q Lines Between Eyebrows.pdf
- N21.009 P1a. Verklaring niet WMO d.d. 09-04-2021[24407].pdf
- Proefpersoneninformatie voor deelname Wenkbrauwlift Versie 2. 17-03-2020.docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Idea for the study: FdJ, SP, KW, LT
Developing study design with appropriate outcome measurements: FdJ, SP, LK, ES, KI, KW, LT
In hospital logistics: FdJ, LK, ES
Drafting the protocol: FdJ, LK, ES, SP, KI, KW, LT
Final approval: FdJ, LK, ES, SP, KI, KW, LT
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
No
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oculoplastic surgery, Orbital Disease and surgery, Lacrimal Disease and surgery, Bio-statistics
At the request of the author(s), this article is no longer under peer review.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Feb 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)