Keywords
NAFLD, clinical pharmacist, patient education, life style recommendations, metabolic comorbidities
This article is included in the Global Public Health gateway.
NAFLD, clinical pharmacist, patient education, life style recommendations, metabolic comorbidities
The logistic and economic pressure on healthcare systems worldwide have encouraged the incorporation of pharmacists in disease state management services. This is mostly attributed to the fact that pharmacists are one of the most accessible and knowledgeable health care professionals when concerning disease and medication characteristics. Thus they can ensure optimization of acute and chronic patient care.1 The pharmacist has a crucial role in managing many chronic conditions, including heart failure, asthma, chronic obstructive pulmonary disease, diabetes mellitus (DM), hypertension, dyslipidemia, overweight and obesity. Incorporating a pharmacist in the care of such diseases has led to the achievement of target therapeutic goals, prevention and management of drug related problems, and reduction of medication costs.2–9
Non-alcoholic fatty liver disease (NAFLD) includes two pathologically distinct conditions; non-alcoholic fatty liver (NAFL), characterized by simple hepatic steatosis and absence of hepatocyte injury, and non-alcoholic steatohepatitis (NASH), associated with hepatocellular injury with or without fibrosis.10 NAFLD is a health condition with significant morbidity, mortality, and economic burden to the healthcare system.11 In these patients, multiple metabolic comorbidities often exist, such as hypertension, type 2 DM, hyperlipidemia, and overweight or obesity. Complications of NAFLD include liver cell failure, hepatocellular carcinoma and the need for liver transplantation.12 Thus, the goal is not only to treat the liver-specific disease but to manage comorbidities and prevent complications.13 The only FDA-approved anti-NAFLD measure is lifestyle recommendations, including dietary and exercise advice. Thus effective patient education and close follow up is needed. Pharmacists can play a significant role in supporting NAFLD patient education to achieve target anthropometric measures and improve hepatic fibrosis and steatosis.14
Pharmacists have the basic knowledge and clinical skills, including prioritization and individualization of therapeutic goals and professional communication abilities, necessary to perform such roles. Unfortunately, clinical pharmacists are struggle to implement their roles within a multidisciplinary team providing patient care in developing countries.15 To our knowledge, studies investigating the impact of pharmacists’ roles in the management of NAFLD are absent worldwide and most of the evidence evaluating the impact of health professional in managing metabolic morbidities focus on physicians and nurses.16 Moreover, evidence supporting pharmacist involvement in NAFLD or metabolic morbidities prevention or management are completely absent in Egyptian population.17 We previously reported the beneficial outcome of adjuvant phosphatidylcholine in Egyptians with NAFLD plus the cornerstone management modality of life style and exercise education conducted by a clinical pharmacist to all study participants.18 As an extension of the previous work, the aim of the current study was to evaluate the impact of a clinical pharmacist education, counseling and follow up in the management of Egyptian NAFLD patients with metabolic co-morbidities.
This non-randomized interventional study was conducted in the out-patient clinics of the tropical medicine departments at Ain Shams University Hospitals and Ain Shams Specialized Hospital in Cairo, Egypt, between January 2016 and January 2019.
Patients older than 18 year with a diagnosis of NAFLD (including NAFL and NASH) according to the EASL-EASD-EASO Clinical Practice Guidelines19 were included in the study. Recruitment was done through tropical medicine physician appointments for ultrasonography. Patients were excluded from the study if they have an evidence of other types of liver disease (alcoholic, autoimmune, carcinoma), have end stage liver disease, were pregnant or lactating, and had used a lipid lowering or weight loss medication in the last 6 months.
Due to the reporting of multiple primary outcomes a number of least 100 patients for both arms together were considered sufficient to achieve an alpha error of 5% and a beta error of 1%. Patients who consented for any of investigations aim were included in statistical analysis to enhance the significance of results.
Since the approval was released on the basis of supporting lifestyle modifications as counseled and followed up a clinical pharmacist to all study participants,18 grouping for statistical analysis was done at the end of the study duration based on patients compliance to scheduled sessions and resulted in the existence of 61 and 41 participants in compliant and noncompliant group respectively.
The study protocol was approved by the institutional review board of Ain Shams University according to the declaration of Helsinki. The study was registered in ClinicalTrials.gov under the identifier NCT04411862. Participants were only recruited after they returned a signed informed consent form.
The interventing clinical pharmacist (the corresponding author) interviewed all eligible participants (n=102) to collect contact details (for follow up), histories, including medical, medication, family, and social histories. All eligible participants were then provided with regular (one every two weeks for six months) health education, counseling and follow up sessions as individualized appointments with the intervening clinical pharmacist in a private consultation room inside out-patient clinics area. The education sessions included an explanation of what NAFLD is and that it is reversible with lifestyle change, the expected complications of inappropriately managed disease and the recommended life-style modifications.37 General advice included aerobic exercise (walking for 30 minutes daily, or > 3 km/day three times weekly) and restricting caloric intake to less than 30 kcal/kg/day while maintaining a balanced diet that included low levels of saturated and trans-fats to achieve gradual weight loss. The target weight loss was a 10% loss within 6 months to avoid rapid weight loss (> 1.6 kg/week) that could increase the progression of NAFLD. Self-regulation was encouraged through participants counselling about regular body weighing, using a smartphone app or internet websites, or even a dairy for counting daily calories, and use of pedometers or body activity trackers. Additionally, a WhatsApp group including all participants and the intervening clinical pharmacist was established. Replies to participant’s inquiries were provided through this group as well as through the interventing clinical pharmacist’s email (provided to patients) for providing individualized patient counseling. The clinical pharmacist also provided participants with a written scheduled appointments for sessions, and educative brochures describing the nature and complications of NAFLD and comorbidities such as diabetes, obesity, and metabolic syndrome, as well as individualized monthly dietary plans for all recruited patients. Participants were encouraged to provide a food and exercise log.20
Participant recruitment, grouping, intervention and follow up are summarized in the CONSORT flow chart (Figure 1).
Participants were grouped according to their compliance to the follow up and education sessions: compliant group, those who attended all 12 sessions (n=61); and non-compliant group, those who attended more than 8 sessions but did not complete all 12 sessions (n=41).21 No participants attended less than 8 sessions.
Study outcomes were measured at baseline and endpoint in both groups. The primary endpoint for NAFLD was the change of NAFLD radiological parameters measured by abdominal transient elastography, using FibroScan® Expert 630 (Echosens, Paris, France) to measure liver fibrosis and steatosis. Liver fibrosis grades included: F0 referring to no fibrosis, F1 referring to portal fibrosis without septa, F2 referring to portal fibrosis with few septa, F3 referring to numerous septa without cirrhosis, and F4 referring to cirrhosis.22 Liver steatosis grades included: S0 referring to <5% steatosis, S1 referring to a 5-33% steatosis, S2 referring to a 33-66% steatosis, and S3 referring to >66% steatosis.23
The primary endpoint for metabolic co-morbidities was the number of patients with MetS defined by the presence of any three out of the following five features: waist circumference in the horizontal plane midway between the lowest ribs and the iliac crest24 ≥102 cm in men or ≥88 cm in women; triglycerides (TG) ≥150 mg/dL; high density lipoprotein-cholesterol (HDL) <40 mg/dL in men or <50 mg/dL in women; blood systolic pressure ≥130 or diastolic >85 mm Hg measured according to the standard technique using validated cuff-based automated devices in a properly configured setting25 or on antihypertensive drug in patients with a history of hypertension; and fasting blood glucose (FBG) >100 mg/dL or on drug treatment for elevated glucose (including diabetics).26 TG, HDL, and FBG were measured by enzymatic colorimetric method with the Olympus Corp. AU 600 autoanalyser using reagents from Olympus Corp. (Hamburg, Germany).
Secondary outcome measurements included anthropometric changes to body mass index (BMI, calculated as weight divided by squared height; weight and height were measured with a Health-ometer professional scale), waist and hip circumference, biochemical markers of NAFLD, and comorbidities such as liver functions (determined by colorimetric assay using kits obtained from Biomerieux Vitek, Inc Missouri USA), lipid profile, and fasting insulin (measured by chemiluminescent immunoassay method; Immulite 1000 System, DPC, Los Angeles, CA; 90045-5597) and blood glucose. Additionally, NAFLD fibrosis score (a combined biochemical and clinical score calculated using platelet count, serum aminotransferases and albumin, age, BMI, and diabetic status, calculated using NAFLD fibrosis score calculator) that estimate the probability of liver fibrosis,23 and homeostasis model assessment—insulin resistance (HOMA-IR; a biochemical score calculated using serum fasting blood glucose and insulin) score that assess insulin resistance in non- diabetic patients27 were estimated for each participant using the HOMA2 Calculator (University of Oxford, 2019). NAFLD fibrosis score categories includes: low cutoffs (<-1.455), predicting a fibrosis level of F0-F2; indeterminate cutoffs (-1.455 to 0.675); and high cutoffs (>0.675) predicting a fibrosis level of F3-F4.13 A HOMA-IR >3 indicates the presence of insulin resistance.27
Recorded data were analyzed using the SPSS, version 20.0 (SPSS Inc., Chicago, Illinois, USA, RRID:SCR_002865). Data were explored for normality using the Kolmogorov-Smirnov test.28 Quantitative data were expressed as mean± standard deviation if parametric and as median and interquartile ranges if non-parametric. Qualitative data were expressed as frequency and percentage. Independent-samples t-tests were used to compare two samples of quantitative parametric means. Post-hoc comparisons were conducted using the Bonferroni correction method. The chi-square test was used to compare qualitative parameters. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant if <0.05 and highly significant if <0.001.
The mean levels and comparisons of selected baseline demographics, weight and metabolic co-morbidities of studied groups are shown in Table 1. The study cohort (n=102) included 86 women and 16 men. There was no statistically significant difference between both groups regarding any of the compared demographics.
| Parameter | Compliant group (n=61) | Non-compliant group (n=41) | Test coefficient | p-value* |
|---|---|---|---|---|
| Age (years); mean ± SD | 51.77±7.72 | 51.22±6.74 | t=0.371 | 0.711 |
| Gender | ||||
| Female; number (%) | 49 (80.3%) | 37 (90.2%) | x2=1.823 | 0.177 |
| Male; number (%) | 12 (19.7%) | 4 (9.8%) | ||
| Weight (kg): mean ± SD | 94.52±10.30 | 98.32±10.79 | t=-1.793 | 0.076 |
| Diabetics; number (%) | 30 (49.2%) | 16 (39.0%) | x2=1.021 | 0.312 |
| Hypertensive; number (%) | 24 (39.3%) | 24 (58.5%) | x2=3.625 | 0.057 |
| Use of hepatoprotectors**; number (%) | 46(75.4%) | 31(75.6%) | x2=0.204 | 0.651 |
| Use of antioxidant | 35(57.37%) | 22(53.65%) | x2=0.1375 | 0.710 |
The compliant group showed a statistically significant higher number of patients with normalized waist circumference (p=0.004, Figure 238) and an improved BMI category, as shown in Table 2. These results were accompanied by a decrease in participant weight in kg in both groups but this was more significant in the compliant group (p=0.003) and confirmed with the percent change of 11.66%±5.77 in compliant versus 8.39%±6.05 in non-compliant group; p=0.007).

NAFLD; non-alcoholic fatty liver disease.
*Statistically significant difference p≤0.05 as compared to non-compliant.
| BMI categories* | Compliance Group (n=61) | Non Compliance Group (n=41) | x2 | p-value** |
|---|---|---|---|---|
| Baseline | ||||
| Overweight | 5 (8.2%) | 0 (0.0%) | ||
| Obese I | 21 (34.4%) | 9 (22.0%) | 6.525 | 0.089 |
| Obese II | 25 (41.0%) | 21 (51.2%) | ||
| Obese III | 10 (16.4%) | 11 (26.8%) | ||
| Endpoint | ||||
| Normal | 4 (6.6%) | 0 (0.0%) | 13.688 | 0.008* |
| Overweight | 15 (24.6%) | 4 (9.8%) | ||
| Obese I | 32 (52.5%) | 18 (43.9%) | ||
| Obese II | 9 (14.8%) | 17 (41.5%) | ||
| Obese III | 1 (1.6%) | 2 (4.9%) |
In the compliant group, at the end of the study, there were significantly lower serum levels of low-density lipoprotein (LDL in mg/dl calculated by Friedewald’s formula; p=0.009), and very low-density lipoproteins (VLDL in mg/dl; p≤0.001). LDL decreased by a mean percent change of 19.93% from baseline in the compliant group compared to 11.41% in the non-compliant group, with a statistically significant difference between groups (p=0.002). The number of patients that reached normal LDL levels (<130 mg/dl) was not significantly higher in the compliant group (p=0.136; Figure 338). TG decreased by a significantly higher mean percent change of 16.48% from baseline in the compliant group compared to 6.24% in the non-compliant group, with a statistically significant difference between groups (p<0.001).HDL (mg/dl) increased by 18.57%±15.18 in the compliant group and 16.40%±19.01 in the non-compliant group but was significantly higher (p=0.010) in compliant group at the endpoint. At the end of the study, significantly higher percentage of compliant participants attained a normalized HDL (>50; p=0.004), and normalized serum total cholesterol levels (<200 mg/dl; p=0.002; Figure 3). At the end of the study, there were no significant differences between studied groups regarding FBG (mg/dl; p=0.209), fasting insulin (FI; μU/ml; p=0.179), HOMA score (p=0.193), serum albumin (P=0.052), AST (P=0.115) and ALT (p=0.405). Also the percentage change from baseline was non-significant for these variables in the compliant group (p=0.073 for FBG, p=0.221 for FI, and p=0.089 for HOMA score).
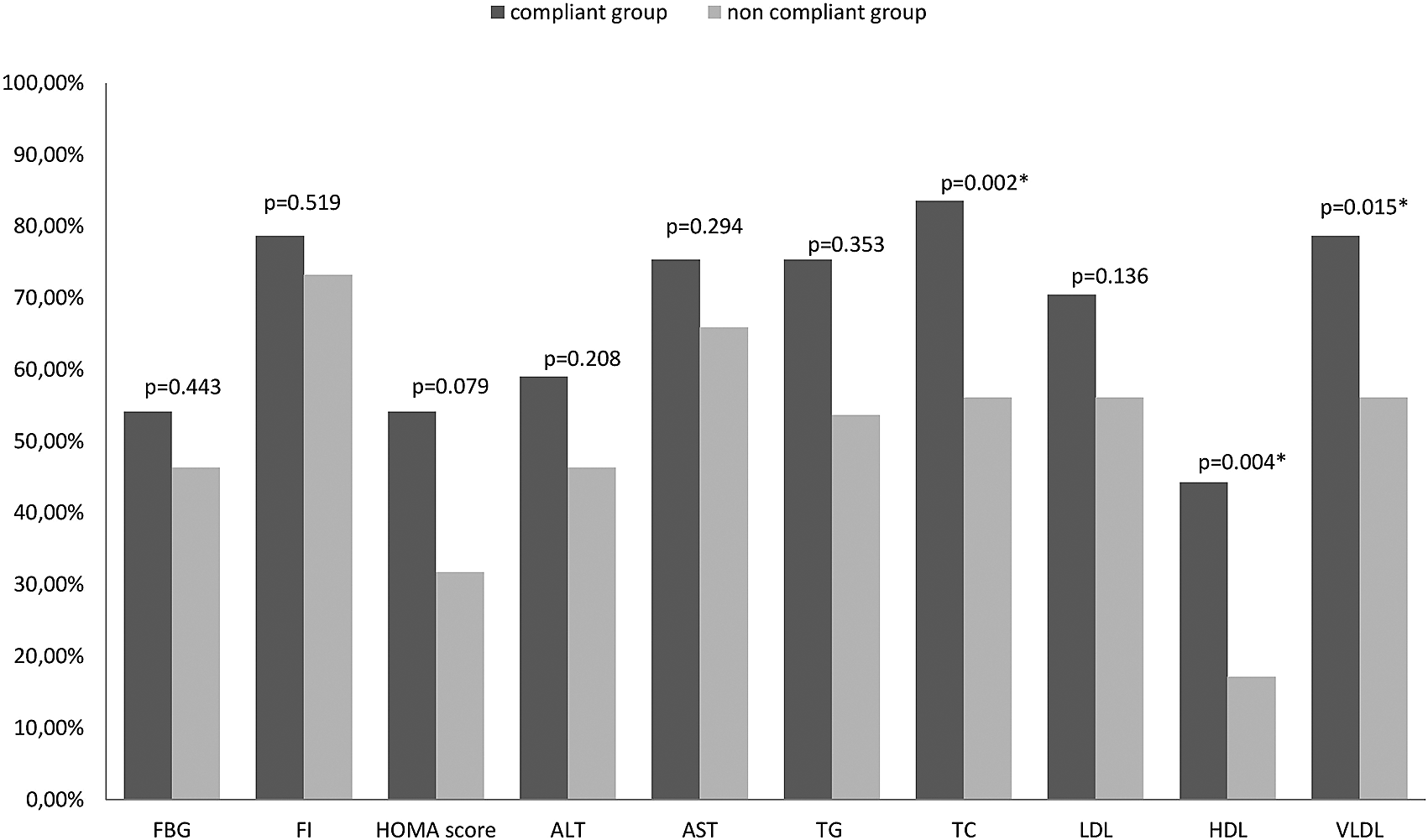
ALT; Alanine Aminotransferase; AST: Aspartate Aminotransferase, FBG: Fasting Blood Glucose; FBI: Fasting Blood Insulin; HOMA-IR: Homeostasis Model of Assessment-Insulin Resistance; HDL-C: High density Lipoproteins-cholesterol; LDL-C: Low Density Lipoproteins-cholesterol; TC: Total Cholesterol; TG: Triglycerides,
Normal ranges: AST= 10-42 U/L; ALT (N=10-45U/L); TC<200 mg/dl; TG <150mg/dl; LDL <130 mg/dl and HDL; >50 mg/dl.
*Statistically significant difference p≤0.05 as compared to non-compliant.
At the endpoint, as seen in Figure 2,38 the compliant group showed a statistically significant higher number of participants with absent dyspeptic symptoms (p<0.0010), hepatomegaly (p= 0.027) and also with improved NAFLD fibrosis score (p=0.0047), radiological steatosis (p=0.009) and fibrosis (p<0.001) grades.
There is no doubt that disease state management by a multi-specialty-health care team has a multitude of advantages, including reducing the pressure on healthcare systems and attainment of improved health care outcomes both to patients and health care institutions. NAFLD is one of the diseases that can benefit the most of being handled by a multidisciplinary approach and this is due to its multiple comorbidity nature.29 Pharmacists are considered one of the most trustworthy and accessible health care professionals, and are ideally situated to provide patient counseling regarding disease state, life style changes and medication.1,30 Pharmacists have a crucial role in enhancing patient health and medication literacy, thus ensuring patient concordance.17
The AASLD recommendation for management of NAFLD prioritizes weight loss via dietary modification alone or with exercise.31 The impact of managing both NAFLD and metabolic co-morbidities chiefly include improvement of anthropometric and metabolic parameters Previous studies have reported a positive impact of incorporation of a pharmacist in patient care, in terms of achieving desired goals, reverting to non-metabolic syndrome status, and improved lifestyle modification.32–34
In the current study, the more significant reduction in participant anthropometric measures in the compliant group, including weight reduction (p=0.003), shifting to an improved BMI category (p=0.008) and waist circumference normalization (p=0.004), was in accordance with the results of several pharmacist-led interventions. A previous systematic review has evaluated the effectiveness of community pharmacist’ weight management interventions, including dietary and physical activity recommendations and follow up in obese patients with obesity related co-morbidities including diabetes, hypertension, depression and gastroesophageal reflux disease. The review reported a statistically significant weight loss at 6 months in three studies.35 The three studies showed an average weight loss of 5.6 kg, 5.1 kg and 5-kg, all much less compared to the average weight loss of 10 kg in the compliant group and 8.5 kg in the non-compliant group in the current study.
Similarly, in a study that enrolled obese patients with comorbidities including DM, hypertension, and/or dyslipidemia, a six month pharmacist led weight loss intervention resulted in a statistically significant reduction in weight (mean loss of 5 kg representing 4.5% from baseline mean, p<0.001), BMI (p<0.001) and waist circumference (p=0.002).36 This is again less than the findings of the current study, which showed a mean percent weight loss of 11.66%±5.77 in the compliant group and 8.39%±6.05 in the non-compliant group, with a significance of p=0.007.
In a study conducted by Hammad et al (2011) in Jordan, to compare the effect of a pharmacist-physician collaborative practice versus usual care in 199 patients with metabolic syndrome, 39.1% of patients receiving pharmacist recommendations and counseling versus 24.7% of usual care patients were successfully shifted from a status of metabolic syndrome to no metabolic syndrome (p=0.032).32 Similarly, the current study showed a reduction in the number of patients with metabolic syndrome at the end of the study, although this was non-significant (p=0.37).
Concerning the laboratory outcomes there were also some studies that agree with the findings of the current study. The non-controlled study conducted by Cording et al on 115 patients with dyslipidemia receiving treatment with a statin (57%), fibrates (17%) and those receiving no lipid-lowering medications (17%).3 After 12 months, LDL level decreased by 20%, HDL cholesterol increased by 11%, and triglycerides decreased by 19% relative to baseline. Overall, LDL goals were reached in 77% of the patients.3 Similarly, in the current study, a significantly higher number of compliant participants converted to normal serum total cholesterol levels compared to non-compliant participants (p=0.002) at the end of the study. The results of the current study are also in accordance with those obtained by Hammad et al, where the mean TG serum level declined by 30.9 mg/dl in the intervention group and by 14.5 mg/dl in the usual care group (p=0.029).32
The positive radiological outcomes of NAFLD obtained in the current study by the pharmacist led counseling and education can’t be compared to similarly conducted studies, as to our knowledge there are none. The higher rate of therapeutic goal achievement in the current study and similarly conducted studies could be attributed to the quality time and individualized attention spent in group and individual patient education, counseling and follow-up that ensured better patient understanding of the disease process, possible complications of inappropriately managed disease and necessary lifestyle changes to manage the disease.
The cost effectiveness of providing this service was not evaluated in the current study. Highlighting the potential long-term healthcare cost reduction secondary to clinical pharmacist implementation into multidisciplinary health team in the face of the expenses of recruiting clinical pharmacists, as well as the identifying the acceptance rate of pharmacist role by non-pharmacist professionals, can reinforce clinical pharmacist collaboration. Future larger scale research is warranted to evaluate the impact of clinical pharmacist collaboration in the management of NAFLD and metabolic morbidities.
The current study has provided significant evidence of the benefit of incorporating a clinical pharmacist in NAFLD patient counseling, education and follow up. This significantly facilitates reaching desired therapeutic goals of NAFLD and metabolic co-morbidities. Thus, the current study indicates that clinical pharmacists could be viable health care providers for such a patient population especially in the face of shortage of primary care provider time in developing countries.
Figshare: Dr Nehal DATA.xlsx, https://doi.org/10.6084/m9.figshare.17104499.37
This project contains the following underlying data:
Figshare: Excel sheet of figures https://doi.org/10.6084/m9.figshare.18953414.38
This project contains the following extended data:
Figshare: Health education material and diet schedule for NAFLD https://doi.org/10.6084/m9.figshare.18866309.20
This project contains the following extended data:
Figshare: CONSORT checklist, https://doi.org/10.6084/m9.figshare.18866306
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Written informed consent was obtained from all the patients to participate in clinical research and for publication of the patients’ clinical details. This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Cohen J: A power primer.Psychol Bull. 1992; 112 (1): 155-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacy Practice; Health Policy; Health Care Financing; Quality Improvement
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: pediatric gastroenterology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Gastaldelli A: Insulin resistance and reduced metabolic flexibility: cause or consequence of NAFLD?. Clinical Science. 2017; 131 (22): 2701-2704 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: NAFLD, Metabolic Syndrome, Atherosclerosis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 24 Feb 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)