Keywords
clomiphene citrate, male infertility, nomogram, semen parameters, testosterone level
clomiphene citrate, male infertility, nomogram, semen parameters, testosterone level
Close to 15% of couples will have subfertility, with a male factor contributing to the infertility approximately 50% of the time.1 While there are often reversible factors identified to treat men with subfertility, empiric hormonal therapy has become a popular therapy used to treat men with idiopathic infertility.
Clomiphene citrate (CC) is the most commonly prescribed medication for idiopathic male infertility in the United States.2,3 The use of CC for the management of ovulation predates the use for men, but there are reports dating back to the 1960s about CC use to treat infertile men with oligospermia.4–6 CC is an antiestrogen blocking the feedback of estradiol on the hypothalamus/pituitary axis to increase the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH).7,8 This in turn increases the local concentration of FSH and LH in the testis, reportedly increasing the production of testosterone and sperm.7–9 In a meta-analysis published in 2013, Chua et al., reported that anti-estrogen use for men with infertility resulted in statistically significantly increased pregnancy rates with an OR of 2.42 (95% CI: 1.47–3.94), sperm counts with a weighted mean difference of 5.24 million/mL (CI: 2.12–8.37) and sperm motility of a weighted mean difference of 4.55% (CI: 0.73–8.37%).9 There was heterogeneity in the dose, type (either CC or tamoxifen) and duration of anti-estrogen analyzed for the meta-analysis.
CC is a relatively safe therapy with Guo et al., reporting from a US claims database no increased incidence of side effects in those using CC, although there was a report of an embolic event occurring with CC use.2,10
From these previous reports, there is evidence that the use of CC leads to improvements in semen parameters and pregnancy rates, but the magnitude of the expected response for individual patients is unknown.
The expected magnitude of change in semen parameters is critical in deciding therapies for couples with infertility. Men with total motile sperm counts (TMC) < 5 million generally will be referred for in-vitro fertilization (IVF), while men with TMC > 5 million are candidates for intra-uterine insemination (IUI) and men with TMC > 9 million are candidates to try to conceive spontaneously.1 The potential goals of therapies for men with infertility is not only to achieve a spontaneous pregnancy, but also to “upgrade” semen parameters so couples might be able to conceive with less invasive assisted reproductive technologies (ARTs) (moving from needing IVF to being candidates for IUI).
Our goal was to develop nomograms based on baseline semen, hormonal and patient characteristics to predict the semen and hormonal changes following CC use. This should allow patients and clinicians to predict the individual’s expected changes in semen parameters and hormone levels following CC treatment, in turn allowing clinicians to tailor the fertility therapies and provide specific information to men on their specific expected outcomes.
This prospective cohort study took place at the Murray Koffler Urologic Wellness Centre at Mount Sinai Hospital, Toronto, Canada and was approved by Mount Sinai Hospital Research Ethics Board with approval number 14-0342-E. All participants provided written informed consent for the use and publication of the patients’ data. Patients were enrolled in this study between 2016–2019 and followed up to 2019. Men attending a specialized male infertility clinic were assessed for fertility. Standardized baseline clinical information including demographic information, fertility history, medical/surgical history and medication use was obtained on all men and prospectively recorded. A description of the initial physical finding was recorded for all men (height, weight, testicular size, presence of varicocele by physical examination).
As a part of routine care, men were asked to provide at least two baseline semen samples with two to seven days of abstinence for testing with a standard analysis of the semen samples recorded as per Bjorndahl et al., 2016: sperm count and motility were measured by a standardized and validated Microptics Computer Assisted Semen Analysis Machine.11 The semen testing was performed in one laboratory (FlowLabs, Oshawa, Ontario). A morning blood sample was taken for baseline hormone testing using an established commercial ELISA technique (including FSH (Cobas kit number: 11775863 122), LH (Cobas kit number: 11732234 122), total testosterone (T) (Cobas kit number: 05200067 190) and Estradiol) as per manufacturers protocol (DPC Immulite 2000, Diagnostic Products Corp., Los Angeles, CA).
As part of routine care, the men were then re-assessed in the clinic and were routinely offered empiric therapy with CC 25 mg every other day if no other specific therapies to treat the male infertility were available or planned. The CC treatment was offered independent of hormone values, semen parameters or clinical characteristics of the men. Men were also instructed to use supplements as empiric therapy if they were not already using supplements.
Men were excluded from the study if they had evidence of infections (positive semen cultures), had a normal semen analysis, had a vasectomy or were unable to provide the samples required for the study. The presence of a varicocele was not an exclusion criterion, but men who had a varicocelectomy recently (< 3 months) or during the course of the study, were excluded from the study.
Men taking CC were followed with hormone assays and semen testing three months following the initiation of therapy. Men with full baseline testing and semen/hormone testing at a minimum of three months following the start of CC and who did not undergo any targeted therapy to improve fertility (e.g. varicocelectomy, infection therapy) were included in this study.
Method of nomogram development
The analyses first resulted in descriptive statistics on characteristics of the clinical parameters at baseline and following three months treatment with CC. Continuous variables were summarized using the median and interquartile range (IQR) and compared using the Mann-Whitney U-test.12
For each outcome, the full model included all variables: age, BMI, TT, LH, FSH, semen volume, percent motility, sperm concentration, and sperm morphology. To relax the linearity assumption, cubic spline terms were included for age, percent motility and sperm concentration. The knots were placed at 25%, 50% and 75% points on the distributions. The model was then developed with predictors selected using backward selection methods by minimizing root mean square error (RMSE) evaluated on 500 bootstrap runs.
A zero-inflated (ZI) negative binomial modeling approach was used to model sperm concentration and sperm motility following three months of CC. ZI models were first introduced by Lambert to account for excess zero counts.13 ZI models are two-part models, consisting of both binary and count model following negative binomial (NB) distribution sections in order to account for excess zero counts. In the logit section, the explanations of regression coefficients are similar to those in logistic regression. In the NB sections, the explanations are the same as in the traditional NB regression models. A generalized linear model for the Gamma distribution was used to model testosterone. A linear model was used to model log transformed LH, FSH and semen volume. All models were internally evaluated using 500 bootstrapped samples. The goodness of fit of the models was assessed by RMSE and the predictive probability curve.
All analyses were performed using the R Stats package version 3.4.4. A two-tailed p-value of <0.05 was considered to be statistically significant.
Empiric CC therapy was used for 121 men (Table 1) with a median age of 36 (IQR 33.00–41.00). Following three months of CC therapy, the median T increased from 9.73 (IQR 7.0–12.0) to 16.0 (IQR 13.75–22.0) nmol/L (p < 0.01), median LH increased from 4.90 IU/L (IQR 3.30–6.40) to 6.0 IU/L (IQR 4.60–9.60) (p < 0.001) while median FSH level increased from 6.70 IU/L (IQR 3.50–9.80) to 7.85 IU/L with IQR (5.00–13.00) (p < 0.001). The full data set is available in the Scholars Portal Dataverse.14
There was a significant increase in the sperm concentration with an increase in the median sperm concentration from 2.4 million/mL (IQR 0.10–7.90) to 5.0 million/mL (IQR 0.4–15.6) and an increase in the mean count from 13.01 ± 3.6 million/ml to a mean of 22.12 ± 4.5 million/ml. There was no significant change in sperm motility or semen volume (Table 1). At baseline, 22% of the men were azoospermic, with 33% of these men having sperm in the ejaculate following CC therapy (p = 0.08, Chi Squared test).
A model was developed (Figure 1) which predicted sperm concentration following three months of therapy with CC. Initial sperm concentration, LH, T, age and sperm motility were all independently predictive of the increase in sperm concentration following CC therapy (Table 1). These as well as serum FSH were included to provide a predicted sperm concentration for individual patients (Figure 1) and to provide the probability that the man’s sperm concentration will be > 15million/mL (WHO normal range), total motile count > 5 million/mL (range below which IVF is often recommended) and 0 million/mL (nomogram available at http://riskcalc.org/clomiphene_citrate/). Predictive probability of the model is shown in Figure 2.

The final semen volume was only related to initial semen volume and none of the other tested parameters, so the model developed for final semen volume prediction only used the baseline volume (Table 2). Final motility was most closely correlated to initial motility, with the final model including age, T, semen volume and sperm concentration (nomogram available at http://riskcalc.org/clomiphene_citrate/).
The post-CC testosterone levels were most closely and significantly correlated to the pre-CC testosterone levels (Table 3), but the addition of information on gonadotropin levels and age significantly improved the predictive performance of the model. The nomogram is shown in Figure 3 with the online nomogram available at (nomogram available at http://riskcalc.org/clomiphene_citrate/). Conversely, LH and FSH levels post-CC also correlated significantly to the pre-CC LH and FSH levels respectively and not significantly to other parameters (Tables 4–6).
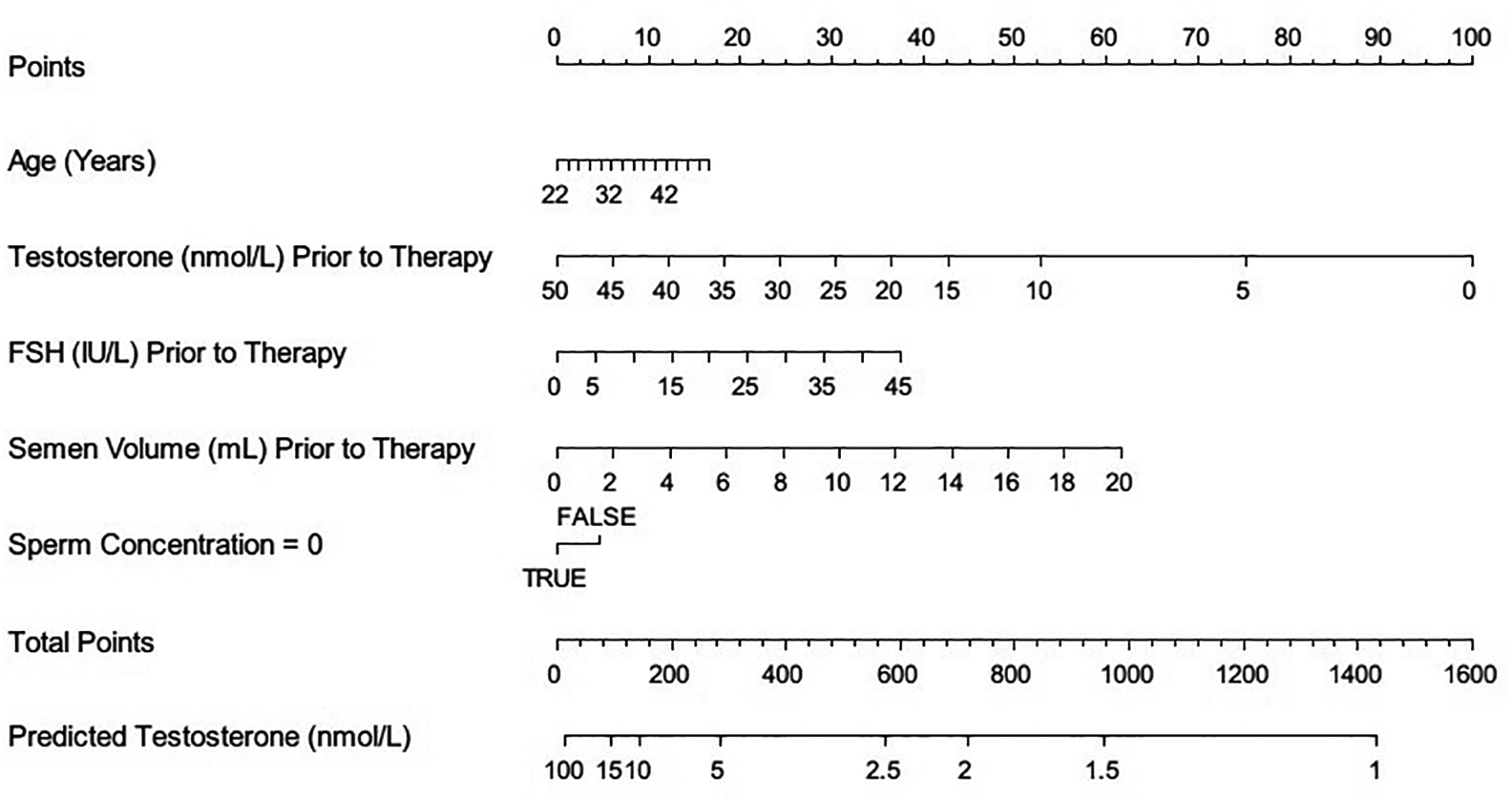
(FSH: follicle stimulating hormone).
| Parameter | Estimate | s.e. | 95% CI | p-value |
|---|---|---|---|---|
| Intercept | 7.157 | 1.029 | 5.118, 9.196 | <0.001 |
| LH.Pre | 0.243 | 0.070 | 0.103, 0.382 | <0.001 |
| FSH.Pre | 0.126 | 0.049 | 0.029, 0.223 | 0.012 |
| SpermConc.Pre > 0 | -2.877 | 0.905 | -4.669, -1.085 | 0.002 |
CC is widely used as an empiric therapy to manage male infertility. Edmund Y. Ko et al., in 2012 reported that CC is the most widely prescribed medication among urologists in the American Urological Association for the treatment of idiopathic male infertility.3
While certainly there are studies which do not show any benefit of CC to treat male fertility, most notably a very large, controlled trial published in 1992,15,16 the majority of studies do show improvements in sperm parameters and pregnancy rates with CC therapy9,17,18 A meta-analysis by Chua et al., published in 2013 found a 2.4 OR (95% CI: 1.47–3.94) of pregnancy in couples where the men were taking empiric CC therapies.9
What remains unclear is the expected response to the CC for individual patients, information which would help couples decide on therapies and allow clinicians to tailor therapies to the individual patient.
With this present study, we have developed a nomogram (available at http://riskcalc.org/clomiphene_citrate/) using baseline values of age, FSH, LH, T levels and semen parameters (volume, count and motility) to provide the expected semen parameters and testosterone levels following CC therapy as well as the odds of having normal sperm counts and total motile counts of > 5 million sperm (level at which IUI could be considered).19 These are the first published nomograms on the expected changes in semen parameters and testosterone levels for individual men following clomiphene citrate therapy.
This nomogram will help clinicians predict the chances of clinically meaningful responses to CC therapy. As an example, a 36-year-old male with semen volume = 1.6 mL, sperm concentration = 7 million/mL and motility = 23% (sperm values where IVF might be recommended), FSH = 3, LH = 2 and T = 12, following CC therapy will have an expected semen volume = 1.6 mL, sperm concentration = 21 million/mL, sperm motility = 21%. There is a 46% chance that his sperm count will be > 15 million/ml (normal) and a 48% chance that the total motile count will be > 9 million. He would have a good chance to increase his sperm parameters to a level where a spontaneous pregnancy is possible.
As another example, a 34-year-old male with baseline semen volume = 1.3mL, sperm concentration = 3 million/mL and sperm motility = 20% (likely needing IVF) with FSH = 3.5, LH = 3 and T = 9. Following CC therapy, the expected semen volume = 1.3mL, sperm concentration = 8.6 million/mL, sperm motility = 20%. Following CC therapy, there is only a 7% chance that his sperm counts will be normal and a 4% chance that the total motile count would be > 9 million. Most likely, this couple would still need to manage the fertility with IVF even after the CC therapy. These are two examples of how this nomogram could help with treatment planning for couples with male factor infertility.
There are several limitations of this study: data comes from a single center using a single CC therapy protocol and should be replicated in other centers, the numbers of patients recruited with azoospermia (due to spermatogenic failure) is low, meaning that our nomogram is likely to be less accurate for men with spermatogenic failure and the men consumed “fertility” vitamins and minerals throughout this study. While guidelines from the American Urology Association and the American Society of Reproductive Medicine conclude that supplements are of “questionable clinical utility in treating male infertility” with non-significant effects on sperm counts, our study can’t preclude an effect of a combination of supplements and CC.20
Paradoxically, the weakness of a single centre is also a strength, with one laboratory for all of the biological testing, which improves the consistency of the testing.
This is the first study to publish a predictive model for expected post therapy sperm parameters in men using CC. This is an important study since it provides clinicians with information to help personalize fertility care for the couples. This type of nomogram will allow clinicians to have a more informed discussion with patients about the expected semen parameters with CC “treatment” and allow couples to make a more informed decision to select between the many options that exist to treat and/or manage (using the Assisted Reproductive Technologies (ARTs)) male infertility.
Finally, in the future, a goal should be to develop personalized predicted pregnancy rates with the use of CC therapy. This would allow clinicians to directly compare the outcomes of CC therapy with the use of the ARTs.
Scholars Portal Dataverse: Underlying data for ‘Nomogram to predict changes in semen parameters following clomiphene citrate therapy for males with abnormal semen parameters’, https://www.doi.org/10.5683/SP2/PMZA4H.14
This project contains the following underlying data:
• Data file: Clomid data – July 2021.tab (Study patients' pre-clomiphene citrate sperm concentration, hormone values and age; and post-treatment 3 months sperm concentration and hormone values.)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Written informed consent for publication of the patients’ details was obtained from the patients.
We thank Kirk Lo and Ethan Grober who supervised this project and who assisted with a presentation at the American Urology Association Annual Meeting 2020.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Ghanem H, Shaeer O, El-Segini A: Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial.Fertil Steril. 2010; 93 (7): 2232-5 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: male fertility and reproductive endocrinology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Feb 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)