Keywords
under-five children, pulmonary tuberculosis, 25-Hydroxy vitamin D, tropical country
under-five children, pulmonary tuberculosis, 25-Hydroxy vitamin D, tropical country
In 2020, WHO reported 638.000 children aged less than 15 years out of 5.8 million people with tuberculosis (TB).1 Indonesia accounted for 824.000 TB cases with 99.000 childhood TB, ranked as the second-highest TB incidence in the world.1 The global TB data in children represent a significant burden, with TB treatment progress in children being slower than overall treatment progress.1 Despite the inconsistent quality of notification data and challenges in TB diagnosis and treatment, various studies in childhood TB has overgrown in recent years.2–5 However, most of the study in childhood TB were related to diagnosis, supplementation, and treatment of childhood TB.4
There have been various studies conducted to analyse the association between vitamin D and TB, mostly in adults, while studies in children are still lacking. 6–19 According to a study conducted in Indonesia, despite year-round sun exposure, nearly one-third of primary school-aged children had low vitamin D levels.20 Another significant study conducted in Indonesia discovered no link between vitamin D level and the occurrence of latent tuberculosis in children under the age of five.21 Globally, studies have found an association between a low level of vitamin D and less immunity of the host against tuberculosis infection.14,22 Indonesia has an abundant sun exposure that theoretically has an essential role in the formation of vitamin D. Therefore, evaluating vitamin D in children actively living in tropical country will be practically significant. This study evaluates the vitamin D level in under-five children with pulmonary TB compared with healthy children control group.
This comparative study with a cross-sectional design was conducted at Rumah Sakit Paru Provinsi Jawa Barat (Lung Hospital of West Java Province) from February 2019 – February 2020. The study protocol was approved by the Research Ethics Committee of the Universitas Padjadjaran with the letter number 0818101481. The inclusion criteria were children under five years old, newly diagnosed with pulmonary TB and have not receive antituberculosis therapy. Prior to participating in this study, all subjects’ parents provided written consent. In this study, the 95% confidence level (Zα = 1.65 one-sided test) and 80% power test (Zβ = 0.84) were selected. The calculation of the sample size determined 35 people for each group.
Diagnosis of TB case was performed by using the scoring system of the Indonesian Pediatric TB Scoring System based on history taking, common tuberculosis clinical findings such as fever and cough, laboratory (tuberculin skin test, gram staining from sputum or gastic lavage) and radiology (chest X-ray) results. Scoring ranges from zero to three for each variable; score of more than six from a fourteen maximum score are considered as tuberculosis diagnosis.23 The isolation of Mycobacterium tuberculosis using nucleic acid testing was conducted in this study using sputum induction technique.
The selection of control group subjects was carried out in age and gender who lived in the same environment as TB subjects, did not meet the tuberculosis criteria based on the scoring system, and had negative tuberculin test. We excluded children with liver or kidney abnormalities, immune-compromised, and children who already received vitamin D supplementation. Consecutively, subjects for the TB group were selected until a minimal sample size was obtained. After obtaining written consent, all subjects had their blood specimens drawn to measure 25-hydroxy vitamin D serum using the enzyme-linked immunosorbent assay (ELISA) method. The 25-hydroxy vitamin D (25(OH)D) is the most frequent metabolite in circulation and its measurement is the barometer of vitamin D status.24 In this study, vitamin D deficiency is defined as serum 25-hydroxyvitamin D levels less than 50 nmol/L, insufficiency is defined as values between 50 and 75 nmol/L, and normal levels are greater than 75 nmol/L.14 The primary outcome measures in this study are the comparison of 25-hydroxyvitamin D serum level in children with pulmonary TB and control group.
Bias is avoided by establishing stringent inclusion and exclusion criteria, particularly for subjects in the control group. Proximity to the researcher, close interaction with other subjects, and parental expectations of involvement in the study had no influence on the selection criteria. There were no missing data in this study because data collection occurred only once during the subject’s assignment as part of the study.
The confounding factors in this study included milk intake and nutritional status analyzed with the chi-square and Fisher Exact test. All data obtained were recorded and tabulated, then an analysis was performed to compare the differences in 25-hydroxy vitamin D mean by the independent t-test. Values of p<0.05 were considered statistically significant. All calculations were assisted with SPSS version 17. The cutoff values for 25-hydroxy vitamin D levels, sensitivity, and specificity were determined using receiver operating characteristic (ROC) curve analysis.
We assessed 83 patients for eligibility to participate in the study: 41 were assessed for TB group and 42 were assessed for control group. In TB group 5 patients were ineligible and one patient declined to participate. In control group 2 patients were ineligible and five patients declined to participate. The flowchart is shown in Figure 1. The study included 70 children who met the inclusion criteria and consent to participate, 35 patients for each group. Table 1 shows the overall characteristics of the research subjects.
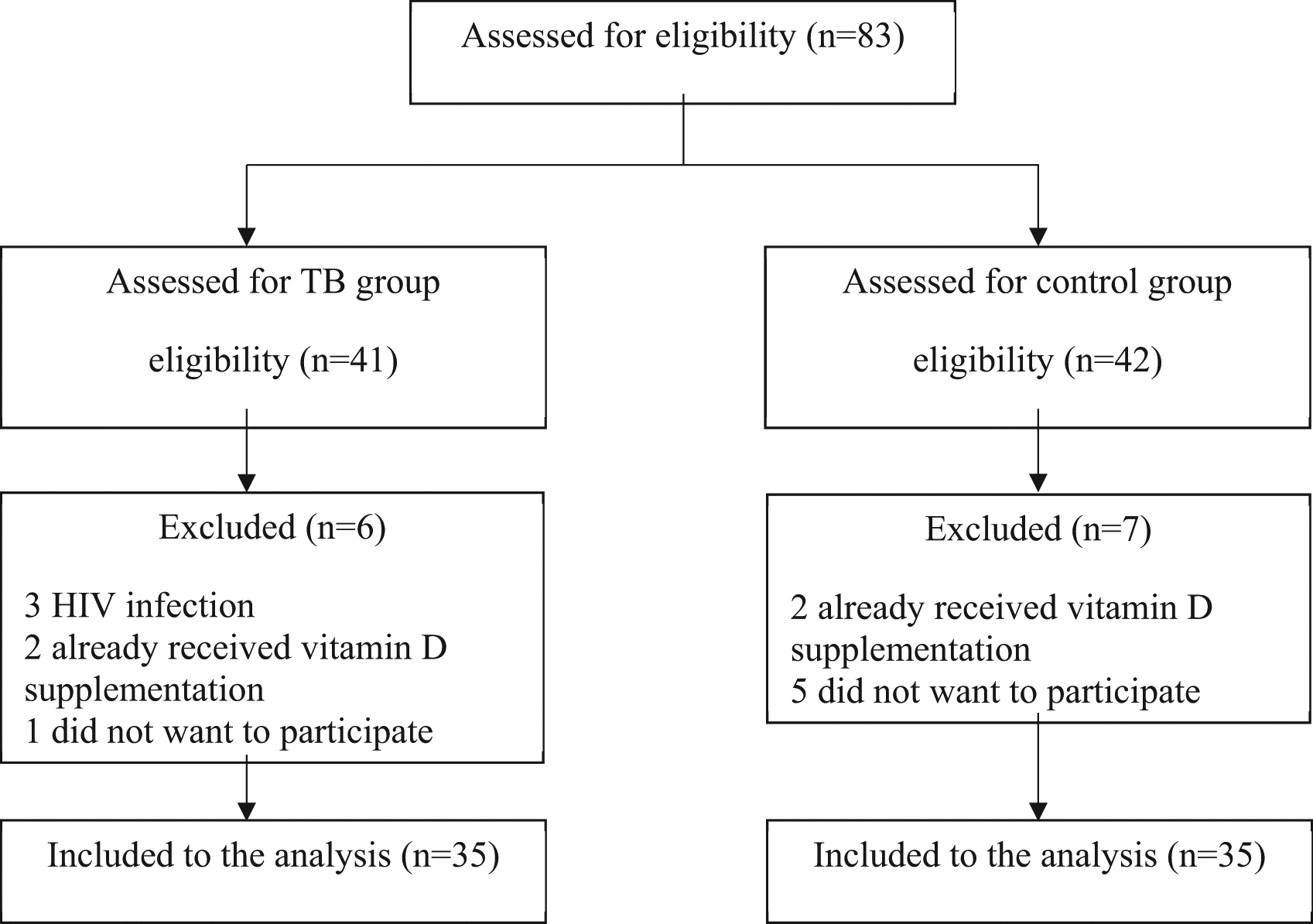
Abbreviations: TB, Tuberculosis; HIV, human immunodeficiency virus
| Characteristics | Tuberculosis group (n=35) | Control group (n=35) | P |
|---|---|---|---|
| Sex | 1.0* | ||
| Male | 17 | 17 | |
| Female | 18 | 18 | |
| Age (months) | 0.167** | ||
| Mean (SD) | 35.8 (15.9) | 30.8 (13.8) | |
| Median | 37 | 33 |
Table 1 shows that the number of boys and girls in both groups was equal. The TB group had a mean age of 39.4 months, while the control group had a mean age of 30.8 months. Subjects in the TB group were newly diagnosed children under five years old with pulmonary TB and have not receive antituberculosis therapy. Thirty-five children with pulmonary TB had a score of more than six based on the scoring system of the Indonesian Pediatric TB Scoring System (≥6 from 14 maximum) and positive tuberculin skin test. Chest x-ray results showed enlargement of lymph nodes from 33 children (94.28%), and two children (5.72%) showed miliary TB. Two children were confirmed to have miliary TB based on the positive results on sputum smear and sensitive Xpert MTB/RIF. The other 33 TB children had negative results on sputum smear and undetected MTB on Xpert. The basic characteristics of the subjects for both group were sex matched and the age difference were statistically insignificant (p=0.167).
Factors related to vitamin D levels assessed in this study included milk intake and nutritional status. Most subjects (88.57%) in both groups received formula milk consumed every day. In the TB group, 57.14% of subjects had moderate or severe malnutrition, and 42.86% of subjects were stunted. All subjects in the control group had good nutritional and height status. Most of the research subjects, both in the control and TB groups, came from families with lower-middle economic backgrounds. The association between milk intake, nutritional status, and height status is shown in Table 2.
| Risk factors | Tuberculosis group | Control group | p |
|---|---|---|---|
| Milk intake | 1.0** | ||
| Breastfeed | 4 | 4 | |
| Formula | 31 | 31 | |
| Nutritional status | <0.001* | ||
| Good nutrition | 15 | 35 | |
| Moderate malnutrition | 15 | 0 | |
| Severe malnutrition | 5 | 0 | |
| Height status | <0.001** | ||
| Normal height | 20 | 35 | |
| Stunted | 15 | 0 |
Table 2 shows there is no correlation between vitamin D levels and milk intake (p> 0.05). Based on that result, milk intake is not considered as confounding factor. There is a significant correlation between nutritional status, height status, and vitamin D level (p<0.001). Table 3 displays the independent t-test results for differences in vitamin D levels between the two groups.
| Vitamin D (nmol/L) | Tuberculosis group (n=35) | Control group (n=35) | p |
|---|---|---|---|
| Mean (SD) | 42.7 (20.2) | 97.7 (10.3) | <0.001* |
| Range | 7.6 – 80.0 | 73.2–123.3 | |
| Vitamin D status | <0.001** | ||
| Normal | 1 | 34 | |
| Insufficiency | 12 | 1 | |
| Defficiency | 22 | 0 |
The mean vitamin D level in the TB group was 42.7, with a range of 7.6-80.0, whereas the mean vitamin D level in the control group was 97.7, with a range of 73.2-123.3. In the control group, none of the subjects experienced vitamin D deficiency. In the TB group, 23 of 35 children (65.71%) had vitamin D deficiency and others (34.29%) had vitamin D insufficiency. Vitamin D levels in the TB group were substantially lower than in the control group (p<0.001), this findings was based on the t-test results.
Based on Figure 2 of the receiver operating characteristic (ROC) curve analysis, the optimal vitamin D level cutoff values for TB were ≤80 nmoL/L, with sensitivity, specificity, PPV, and NPV of 100%, 97.1 percent, 97.2 percent, and 100%, respectively (area under the curve [AUC]: 0.998; Figure 2).
This research is the first study in Indonesia to evaluate vitamin D level in under-five children with pulmonary tuberculosis actively living in tropical country. In this study, vitamin D levels in TB group were significantly lower compared to healthy children in the control group. This result align with systematic review result done by Sutaria.25 Previous studies conducted by Venturini et al. with subjects aged <18 years, in children with latent and active TB, showed that hypovitaminosis D was significantly associated with TB infection.14 Yani et al. discovered that the incidence of latent tuberculosis was 1.44 and 1.67 times greater in patients with vitamin D insufficiency and deficiency, respectively, than in subjects with adequate vitamin D.21
Factors affecting vitamin D levels include genetics, geographical location, tropical climate, skin color, race, food intake, and exposure to sunlight.14,26 A typical symptom in children with tuberculosis is a loss of appetite, which leads to a drop in nutritional intake and a low level of vitamin D. Malnutrition has been connected with an increased risk of TB. Nutrition has a vital role in generating proper innate immune responses to TB. Specifically, vitamin D itself is essential for downstream gene expression important for the immune response against Mycobacterium tuberculosis. The result of this study shows similar result with available study that nutritional status affects the risk and progression of tuberculosis.27
Food intake in this study was limited to milk, and other types of foods were not taken into account. There is no significant difference between breastfed milk and formula, so it did not affect the results of this study. Other factor that is not included in this study is determination of vitamin D composition in each brand of consumed milks; although most of the milks in Indonesia are vitamin D fortified, some milks might not fortified with vitamin D. This study did not trace the source of milk consumed by the subjects due to high variability of milks that can be randomly consumed. Although this study did not stratify the respondents’ socioeconomic condition, the majority of the subjects’ parents are uneducated (below the junior high school level) and unemployed or working as informal laborers. As a result, the type and amount of milk consumed can vary substantially depending on the parents’ uncertain economic circumstances, assistance or support from donors, and government engagement at specific times.
It was possible to exclude risk factors such as geographical location (tropical climate), skin color, and race in both study groups, because both groups had the same risk factors. Both groups belonged to the Malay ethnicity and were natives of the West Java region, making them geographically and climatically similar. It is assumed that all participants were under the age of five and lived in the same location, and that there were no practical instruments to measure the wide range of clothing and everyday activities. Because there was no standardized way for assessing the quantity of sun exposure, the amount of sunlight exposure was not measured.28 This study’s findings contrast significantly from those of an Indonesian study of school-aged children, which revealed that one in every three youngsters lacked adequate vitamin D levels.20 Different research locations (rural and urban) and other activities (indoor and outdoor) may have contributed to the level of vitamin D.
The TB subjects in this study met clinical TB criteria, but only two subjects were bacteriologically confirmed. Lack of bacterial confirmation can be caused by difficulties in obtaining a bacterial sample. This condition leads to a burden in assessing TB diagnosis on under-five children. Our study shows that examination of vitamin D levels carried out by taking blood samples can be considered as supplementary in TB diagnosis (p<0.001, sensitivity 100% and specificity 97.1%) because children with TB significantly have low vitamin D levels. These findings can encourage further research for the use of examination of serum vitamin D levels to determine hipovitaminosis D as a risk factor for tuberculosis in under-five children. Currently, the examination of serum vitamin D levels cannot be done at an affordable cost, but with the great challenge in establishing the diagnosis of TB due to the difficulty of sample extraction in under-five children, the development of cost effective examination technique for serum vitamin D levels can be an alternative modality.
A previous study in adults showed that sufficient level of vitamin D as protective factor against TB in adult, while low serum of vitamin D can increase the progression to TB up to 5-fold increased risk.29–31 There is limited study in under-five children to conclude those same protective and risk factors of vitamin D level against tuberculosis. A study in the United Kingdom with child subject suggest a high utilization of vitamin D which served as a host defense mechanism towards Mycobacterium tuberculosis, starting from the incubation period until the development of disease.32 This proposition might support the hypotheses of vitamin D as protective agent in tuberculosis and the mechanism of vitamin D high utilization can lead to a low level of vitamin D.
This was a cross-sectional study with no subsequent follow-up and assessment of clinical problems, changes in vitamin D levels over time, or any vitamin D status intervention. Additional multi-center studies and monitoring of vitamin D levels throughout tuberculosis treatment may provide additional knowledge about vitamin D and tuberculosis. The number of factors that affect vitamin D is a big challenge in research related to vitamin D status. However, from the existing studies, a significant assumption can be concluded from the low levels of vitamin D in TB cases and an increased risk of progression in latent TB cases to TB.21 The low level of vitamin D in children with TB is an indication that they are given vitamin D in addition to TB drugs. This presented a concern about the significance of vitamin D in boosting the in vitro antibacterial action of TB patients that might operate synergistically with TB medications in eliminating TB.9
In this study serum levels of vitamin D in children with TB were significantly lower than in healthy children. Study with a larger sample size and age stratified are required for providing more profound evidence regarding vitamin D level in tuberculosis. Further research on the use of vitamin D supplementation in the treatment of children with tuberculosis has the potential to make substantial scientific and therapeutic contributions.
Figshare. Dataset TB-N70FIG.xlsx. DOI: https://doi.org/10.6084/m9.figshare.19524229.v233
This project contains the following underlying data:
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a clinician practicing in paediatric infectious diseases and have participated in similar research into the association between vitamin D levels and TB status.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vitamin D, osteoporosis, osteoarthritis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 21 Apr 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)