Keywords
Lead, Pomegranate, Retina, Histopathology, TEM
This article is included in the Agriculture, Food and Nutrition gateway.
Lead, Pomegranate, Retina, Histopathology, TEM
Lead (Pb) is a prominent environmental toxic heavy metal that has adverse effects on human health. Pb and its compounds can easily accumulate in air, water, and soil1. After absorption, Pb is transported to most body organs especially the liver, kidney, brain, and finally accumulates in bone throughout life.2,3 It had been reported that there is no safe dose of Pb exposure and even very low doses have toxic effects on both humans and animals.1 Moreover, prolonged exposure to Pb can cause neurological, hematological, cardiovascular, gastrointestinal, immunological, and fertility disorders.4 During gestation, the maternal stored Pb can cross the placenta by diffusion resulting in adverse effects on the developing fetuses.5 Another study revealed that there is a positive correlation between maternal and umbilical cord blood Pb levels, indicating the transfer of Pb from mother to fetus.6 Also, Pb can transfer to neonates during lactation via the breast milk.7,8 It had been claimed that neonates are more sensitive than adults to Pb.9 The toxic response for Pb depends on several factors, like the dose, the age, the life stage of a woman, duration of exposure, and health and nutritional status of the individual.10 Accordingly, Pb constitutes a persistent public health problem.11 Previous studies claimed that the primary site of action of Pb is the central nervous system (CNS).12,13 Due to the lineal correlation between eyes and the CNS, there is no doubt that the ability of Pb to resist the development of the CNS will also affect eye development in fetuses. Pb exposure leads to reduced sensitivity of photoreceptors, blurred vision and cataracts, and optic neuritis.14
Certain fruits and vegetables contain high levels of phenolic compounds that typically act as natural antioxidants.15 Pomegranate (Punica granatum) is a sweet fruit that have an ancient history and is mentioned in many Holy Scriptures such as the Torah, the Bible, and the Holy Quran.16 The pomegranate fruit has essential polyphenolic compounds in different parts of the fruit that include the peel, seeds, and arils. Other compounds such as organic acids, sugars, minerals and vitamins have been reported in pomegranates by many instigators.17,18 Moreover, the peel part of pomegranate contains bioactive phenolic compounds such as ellagitannins, flavonoids, and proanthocyanidin as well as minerals and complex polysaccharides.19,20 The arils part contains 85% H2O, 10% total sugars, especially fructose and glucose, pectin, organic acids such as malic acid, ascorbic acid, and citric acid as well as phenolic compounds and flavonoids like anthocyanins.18 The pomegranate seeds are rich with total lipids such as linoleic, stearic acid, punicic acid, palmitic acid and oleic acid.21,22 Also, the seeds are rich with protein, minerals, vitamins, pectin, crude fibers, sugars, isoflavones and polyphenols.23
Various medicinal uses have been documented for pomegranate extracts.24,25 The pomegranate is widely used in various medicinal practices such as wound-healing,26 as well as having anti-tumor properties,23,27 anti-atherosclerotic capacities,28 antihepatotoxic agents,29 and in the amelioration of cardiovascular diseases.30 Moreover, it has been documented to have anti-diabetic,25,31 anti-inflammatory,32 and antimicrobial33 activities as well as ameliorative properties for general metabolic health.34,35 Accordingly, the present study was mainly designed to evaluate the potential ameliorative role of pomegranate juice against Pb-induced retinal cell damage in pregnant rats and their neonates.
- Ethical considerations: The ARRIVE guidelines were followed. All efforts were made to ameliorate the suffering of animals. This study was approved by the Bioethics Committee of Damanhur University, no. EA 23122, 2020. All experiments inclusive of animal handling and sacrifice were conducted as per the guidelines of the Bioethics Committee of Damanhur University.
- Equipment: Light microscope fitted with digital canon camera, electron microscope, flow cytometry system (FL2-H), blender, microwave rotatory vacuum.
- Chemicals: Lead acetate (C4H6O4Pb3H2O) in the form of white crystals, GFAP and P53 antibodies were purchased from Sigma Chemical Company, Cairo, Egypt.
Fresh pomegranates fruits were washed, crushed, squeezed using a blender and then treated with pectinase to obtain PJ and by-products. The PJ was filtered, pasteurized, concentrated using microwave rotatory vacuum and atmospheric heating process respectively, and stored at -20°C. 20 mL of concentrated PJ was diluted in 500 mL of distilled water and kept in refrigerator until use. A total of 2.5 mL diluted PJ includes 100 μL PJ, which is equivalent to 2.8 μmol total polyphenols was supplemented to rats each other day.36,37
For this study, 32 Wistar albino rats (24 females and 8 males) were obtained from the Holding Company for Biological Products and Vaccines (VACSERA, Cairo, Egypt). They were 2-3 months old and had an average weight of 180 g. Animals had the same housing conditions of a clean house, regular dark-light cycle, normal ventilation and an adequate, stable, balanced diet with water ad libitum. After an acclimatization period of two weeks, the animals were mated in the special matting cages (1 male: 3 females) overnight. After 3-4 days and ensuring of pregnancy via observation of vaginal plug and using vaginal smear method, pregnant females were separated from males. The pregnant rats were randomly divided into four groups (n=6): group 1, control; group 2, pomegranate juice (2.5ml of diluted PJ, every other day); group 3, lead (oral dose, 18.5 mg/kg B. Wt, each other day);38 and group 4, Pb & PJ (Pb alternatively with PJ). Researchers were aware of the group allocations. Groups 2, 3 and 4 were exposed to the appropriate dose of treatment from the 4th day of pregnancy till the end of weaning. The offspring (six from each group) were weighed postnatal and examined at the ages of one week, two weeks and three weeks. The mothers were also examined and processed at the end of weaning period. The study took place from August 2020 – August 2021. No rats were excluded from the final analysis.
Experimental procedure
Investigated parameters
Data were expressed as mean + standard error, (n=6 per group). Statistical analyses were one-way ANOVA followed by post hoc test, performed in Excel using the data analysis – ANOVA function. Means in the same row with different superscript (*) are significantly different (p>0.05), *significant at p-value>0.05, ** significant at p-value>0.01 and ***significant at p-value>0.001.
Histological sections by hematoxylin and eosin
The animals were euthanized using di-ethyl ether and the eyes of mothers (at the end of weaning) and their neonates (7, 14 and 21 days old, 3 offspring for each age from each group) were removed immediately, washed in normal saline and fixed in 10% neutral buffered formalin.39 After fixation, the eye specimens were dehydrated with ascending grades of ethanol, cleared with xylene, and embedded in paraffin. A 5 μm circular cut sections was made up vertical to the corneal margin and parallel with the optic nerve. The sections of the eye were stained in Mayer’s haematoxylin and eosin and processed for investigation of the retina under bright field light microscope and photographed.
Immunohistochemical labeling of glial fibrillary acidic protein (GFAP) and P53
5 μm thick paraffin sections were cut, mounted onto positively charged slides, de-paraffinized and rehydrated in descending series of alcohol. Endogenous peroxidase activity was inhibited using 3% H2O2 in methanol for 40 min at room temp. The retinal sections were retained at 25°C and processed for antigen retrieval by digestion in 0.05 % trypsin. After thorough washing in Tris buffered saline (TBS), pH 7.6, the sections were treated with normal swine serum and divided into two groups for further incubation with the appropriate primary antibody. For demonstration of GFAP activity, the sections were incubated for 30 minutes with polyclonal rabbit anti- GFAP antibody (1:300 dilutions, DAKO Corporation, California, and USA). After three washes in TBS the sections were incubated for 30 minutes with anti-rabbit antibody raised in swine. After thorough washing with TBS the sections were incubated with horseradish peroxidase-rabbit anti-horseradish peroxidase complex (PAP) for 30 minutes and washed for 30 min. Other sections were processed for demonstration of P53 activity by incubation for 45 min with diluted 1:10 monoclonal primary antibody (Anti-p53; clone DO-7 Dako). Slides were then rinsed in PBS and subsequently incubated in the presence of the secondary antibody for 20 min. For all sections, the complex sites were shown brown using 3, 3 diaminobenzidine tetrahydrochloride with fresh hydrogen peroxide substrate. The sections were counterstained with Mayer’s haematoxylin, mounted and photographed by phase-contrast Olympus light microscope fitted with digital canon camera. Incidences of cellular accumulations of GFAP and p53 protein were determined for each group.
The ultrastructural investigation was mainly focused on the retina of the mother rats and their neonates at 21 days old. Immediately after dissection of the selected animals, small pieces about 1 mm2 of retina were placed in freshly prepared 2.5% cold glutraldehyde as a fixative for about 5 hours. Samples were then washed in two changes of cold phosphate buffer, PH 7.3 for 1 hour. The specimens were post-fixed for 1-2 hours in buffered 1% Osmium tetraoxide, followed by washing in buffer then dehydrated in ascending series of cold ethyl alcohol, cleared in propylene oxide and mounted in epoxy resin. Semi-thin sections of 0.7μm thickness were cut with glass knives on the 6000 MT RMC ultramicrotome. The sections were mounted on glass slides and stained with 0.25% toluidine blue. For the electron microscope preparations, thin sections were cut from a preselected area and mounted in copper grids and stained with lead citrate and uranyl acetate according to.40 The sections were then examined and photographed by a JEOL 1200 EX11 transmission electron microscope in the EM Unit at Mansoura University.
Slices from retinas of all studied groups were taken, and cell suspension was prepared with Tris-EDTA buffer (pH7.4) (Sigma-Aldrich Co.). Cell suspension was fixed in ice-cold 96-100% ethanol (Sigma) at 4 °C overnight, centrifuged at 1,500 rpm for 10 min, and then resuspended in PBS containing 50 μg/mL propidium iodide (PI) (Sigma-Aldrich Co.). For each sample, since analysis was based on measurement of 10000 cells. Single cell suspensions were prepared from retinas from at least six rats of each of the age of the animals, and 1.5-3×106 cells were stained for expression of the designated lineage markers. Cells were harvested by centrifugation, washed in ice-cold PBS, and fixed in 80% ethanol that had been prechilled to 20°C. They were then re-pelleted and re-suspended at a concentration of 0.1-0.3 106/ml in PBS containing 18 mg/ml propidium iodide (PI; Sigma) and 8 mg/ml RNase A (Sigma; PI solution). After incubation in the dark for at least 1 h, cell cycle profile analysis was performed on 10,000-20,000 cells on a FAC Sort flow cytometer using the Cell quest analysis program (Becton Dickinson, Sunnyvale, CA, USA).
The mean body weights of Pb-exposed mothers were significantly lower (p<0.001) than control. On the other hand, the mean body weights of mother rats of group 4 (Pb and PJ) did not appear to be significantly different (p>0.05) compared with the control group. Also, for all studied neonates maternally induced with Pb (7, 14, and 21 days old), the mean body weights were significantly lower (p<0.001) than their corresponding ages of the control. The mean body weights of 7 and 14 day-old neonates of group 4 appeared to be non-significant (p>0.05) in comparison with those of control group, however a significant (p<0.05) decrease in body weight was still recorded in 21 days neonates (Table 1).
| Group (n=6) | Control (G1) | PJ (G2) | Pb (G3) | Pb & PJ (G4) |
|---|---|---|---|---|
| 7days | 17.09±0.19 | 16.56±0.26 | 10.56±0.28*** | 16.33±0.27 |
| 14 days | 27.69±0.34 | 26.98±0.31 | 16.74±0.28*** | 26.60±0.25 |
| 21 days | 35.88±0.38 | 34.71±0.32 | 25.46±0.32*** | 33.78±0.94* |
| Mother rats | 234±1.68 | 227±3.81 | 189±2.87*** | 225±4.36 |
(The software used to edit the images and labels: Adobe photoshopCS8me).
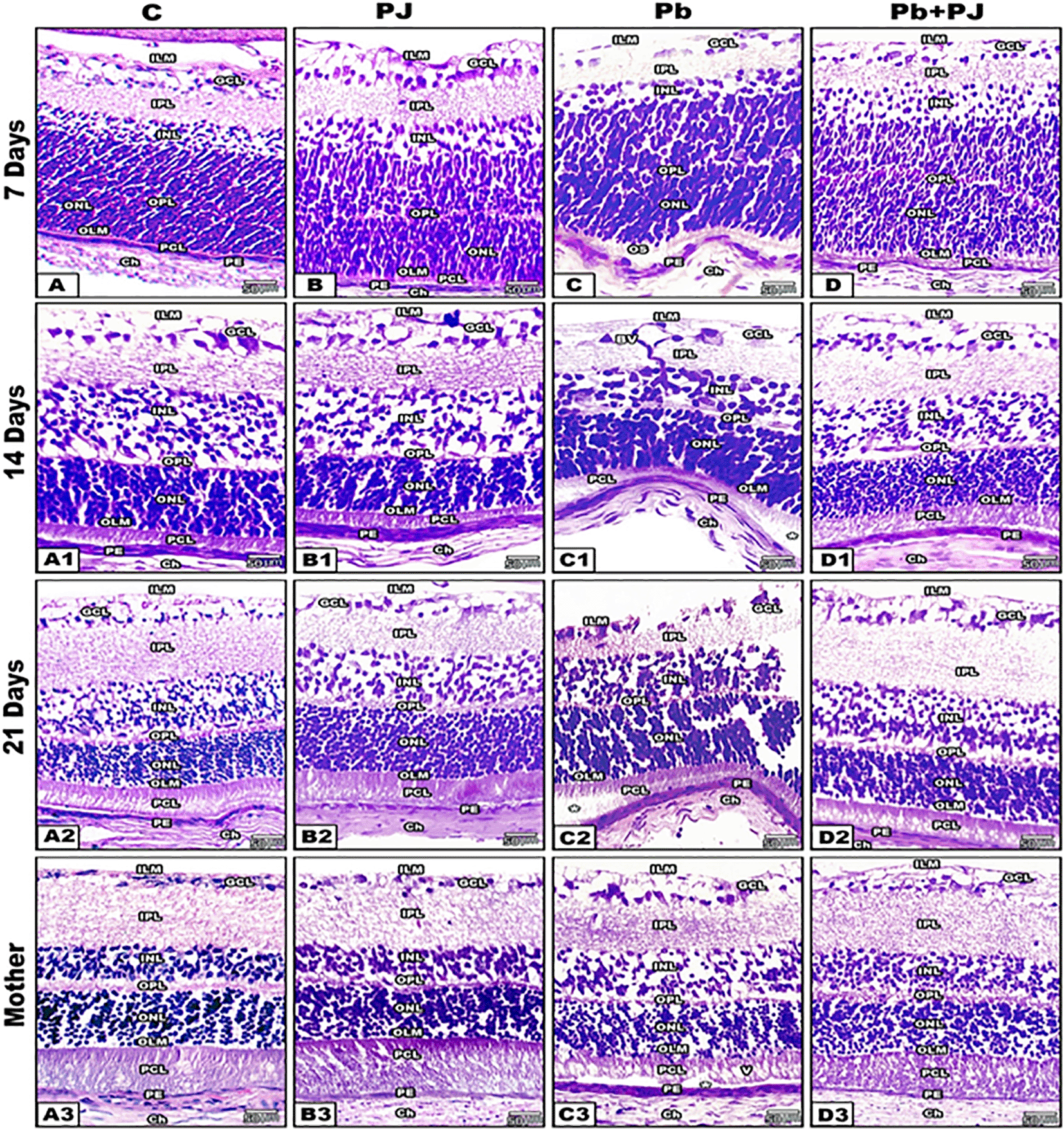
Abbreviations: Inner limiting membrane (ILM), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), outer limiting membrane (OLM), photoreceptors cell layer (PCL), pigment epithelium (PE) and choroid (Ch).
In control and PJ supplemented groups of mother rats, the retinal sections appeared with normal histological architecture. On the other hand, the retinal layers of Pb-treated group showed obvious deleterious histological changes especially in the pigmented epithelium (RPE), photoreceptors cell layer (PCL) and ganglion cell layer, however mild changes were recorded in outer nuclear layer (ONL) and the outer plexiform layer (OPL). The RPE cells appeared hypertrophied with bulged peripheral nuclei. The PCL appeared detached from the RPE with fragmented and vacuolated outer segments. The nuclei of the ONL were aggregated in clusters and separated from the bases of photoreceptors. The OPL appeared relatively thin and disintegrated if compared with control. Moreover, the cells of GCL appeared hypertrophied, highly vacuolated and lost its normal striation pattern.
In Pb group of mother rats co-supplemented with PJ, the retina showed marked amelioration in its histological architecture. Such ameliorations were represented by restoration of retinal detachment and normal arrangement of photoreceptors without vacuoles. The RPE appeared normal with centrally located nuclei. Also, the ONL appeared homogenous and adjacent to the bases of photoreceptors inner segments. The OPL layer appeared compact and in contact with the ONL. The retinal ganglion cells become highly organized in one row and their axons formed a continuous nerve fiber layer.
In the different ages of neonates (7, 14, 21 days old) maternally induced with Pb, the RPE illustrated pronounced wavy and hypertrophied cells, detached outer segments of PCL. On the other hand, marked histological improvement was recorded in the retina of neonates of group 4 (Pb plus PJ) where the outer nuclear layer and the RPE were tight.
Changes in RPE (Figure 2)

(The software used to edit the images and labels: Adobe photoshopCS8me). Abbreviations: BI: basal in folding; BM: Bruch's membrane; CC: choriocapillaris; CP: cytoplasmic processes; DBI: descending basal infolding; DBM: disintegrated Bruch's membrane; GB: Golgi bodies; L: lysosomes; M: mitochondria; N: nucleus; OS: outer segments; Ph: phagosomes; RER: rough endoplasmic reticulum; SER: Smooth endoplasmic reticulum; V: vacuoles; VM: vacuolated mitochondria.
In control and PJ groups of mother rats, the TEM investigation revealed that RPE consists of a single layer of low cuboidal cells which have numerous basal (scleral) infoldings and apical (vitreal) processes enclosing the tips of outer segments of photoreceptors. The cells of RPE appeared rich with smooth endoplasmic reticulum (SER), lysosomes (L), and rough endoplasmic reticulum (RER). In addition, the phagosomes of photoreceptor outer segment are observed.
In Pb-exposed mothers and their neonates, the cells of RPE displayed multiple ultrastructural changes like fragmentized Bruch’s membrane, cytoplasmic vacuolation, vacuolated mitochondria, disintegrated basal infoldings and apical processes as well as loss of RER. Moreover, the nucleus appeared pyknotic with irregular nuclear membranes. On the other hand, the RPE cells of Pb-exposed rats co-supplemented with PJ displayed remarkable improvement in their subcellular architecture. Such improvements were represented by rebuilding of Bruch’s membrane and extension of the cytoplasmic processes in between the photoreceptors’ outer segments. The mitochondria appeared normal with obvious regular cristae. The SER and RER appeared regularly scattered in the cytoplasm especially near the nucleus. Further observation revealed that the nucleus becomes vesicular with their intact membrane.
Changes in Photoreceptors and ONL (Figures 3 and 4)

(The software used to edit the images and labels: Adobe photoshopCS8me). Abbreviations: BM: Bruch's membrane; CP: cytoplasmic processes; DIS: damaged inner segment; FOS: fragmented outer segment; IS: inner segment L: lysosomes; M: mitochondria; MD: membranous disc; N: nucleus; OLM: outer limiting membrane; ONL: outer nuclear layer; OS: outer segments; V: vacuoles.
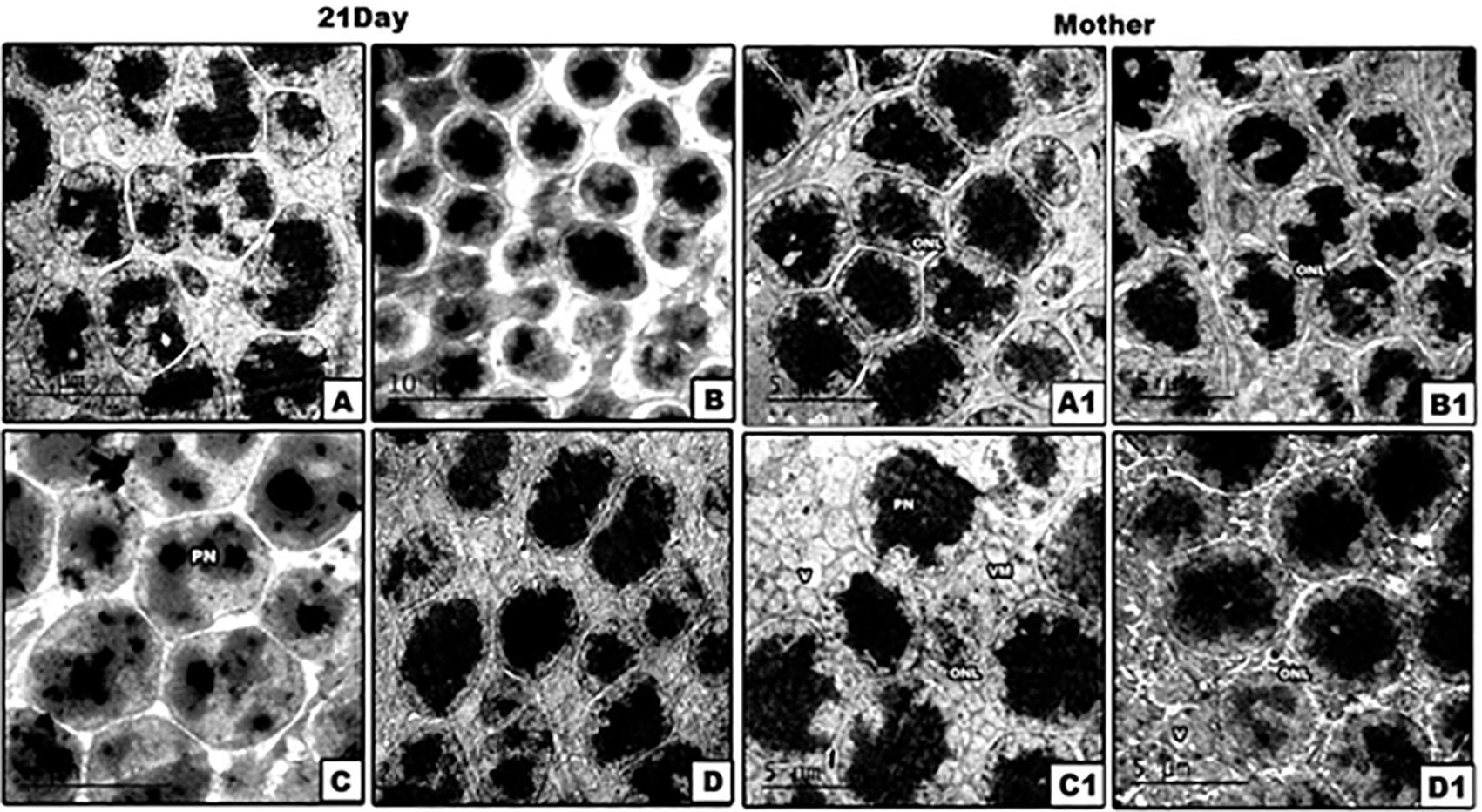
(The software used to edit the images and labels: Adobe photoshopCS8me). Abbreviations: ONL: outer nuclear layer; PN: pyknotic nuclei; V: vacuoles; VM: vacuolated mitochondria.
Our results by TEM revealed that the photoreceptors of control and PJ supplemented mother rats and their offspring appeared regular, and their outer segments interdigitate with the cytoplasmic processes of the RPE cells. The outer segments (OS) of photoreceptors consist of organized membranous discs, however the inner segments displayed rounded to oval nuclei. The outer limiting membrane appeared intact. In Pb treated mother rats, the OS of photoreceptors appeared fragmented and separated from the IS. Furthermore, the IS of photoreceptors were dislocated from the ONL and became aggregated in clusters with pronounced loss of their mitochondria. The neonates of Pb-exposed rats didn’t show any changes in their photoreceptors.
Co-supplementation of PJ to Pb-exposed rats successfully restored the deleterious ultrastructural changes induced by Pb, whereas the OS of photoreceptors displayed a regular pattern of membranous discs and the IS appeared in direct contact with the ONL. In Pb treated mother rats and their neonates, the ONL appeared with multiple pyknotic nuclei and fragmented cell membranes with wide intercellular spaces. Furthermore, the ONL of mother rats and their neonates of group 4 appeared normal with less intercellular spaces.
Changes in INL of retina (Figure 5)
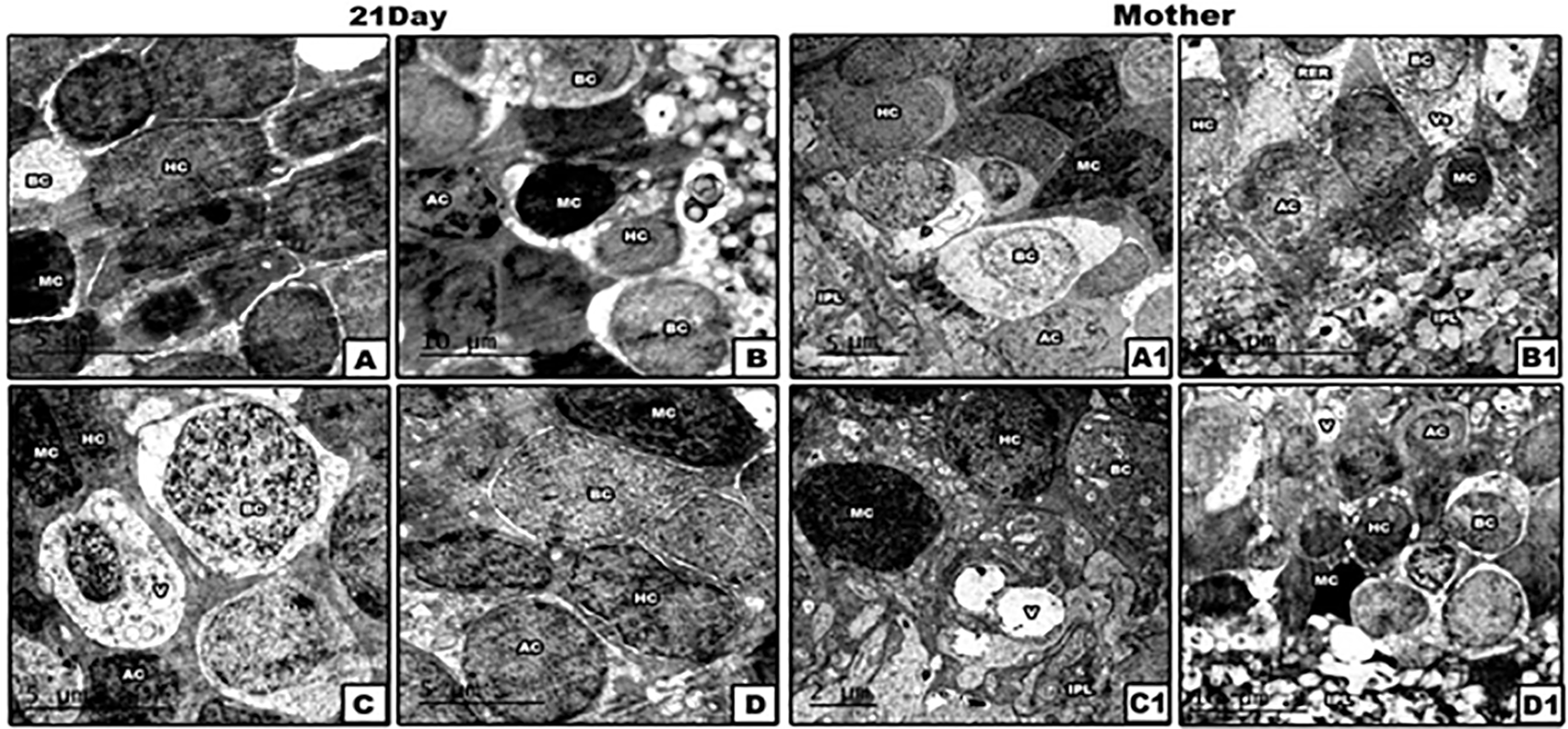
(The software used to edit the images and labels: Adobe photoshopCS8me). Abbreviations: AC: amacrine cells; BC: bipolar cells; HC: horizontal cells; IPL: inner plexiform layer; MC: Muller cells; RER: rough endoplasmic reticulum; V: vacuoles.
Generally, among mammals, the inner nuclear layer is made up of four categories of neuronal cells: bipolar cells (BC), horizontal cells (HC), amacrine cells (AC) and Muller cells (MC). The BCs are numerous, rounded or oval in shape and each is prolonged into an inner and an outer process. The HCs lie in the outer part of the INL and possess somewhat flattened cell bodies. Their dendrites divide into numerous branches in the OPL, while their axons run horizontally for some distance and finally ramify in the same layer. The ACs are localized in the inner part of the INL, they have not yet been shown to possess axis-cylinder processes. Their dendrites undergo extensive ramification in the inner plexiform layer. The MCs display dark, elongated nuclei. The Muller cells form an almost continuous layer separating the sclerally located BC and HC from AC somata are mostly positioned at the vitreal side of the inner nuclear layer.
In lead acetate treated mother rats and their neonates, the INL exhibited marked degeneration of horizontal cells, bipolar cells and amacrine cells. Such cellular degeneration was represented by disintegration of most cytoplasmic organelles, nuclear pyknosis and damage of cell membranes. Furthermore, the cells of the INL of Pb-exposed rats post-supplemented with PJ (group 4) appeared normal with less vacuolation still found in the cytoplasm in some cells.
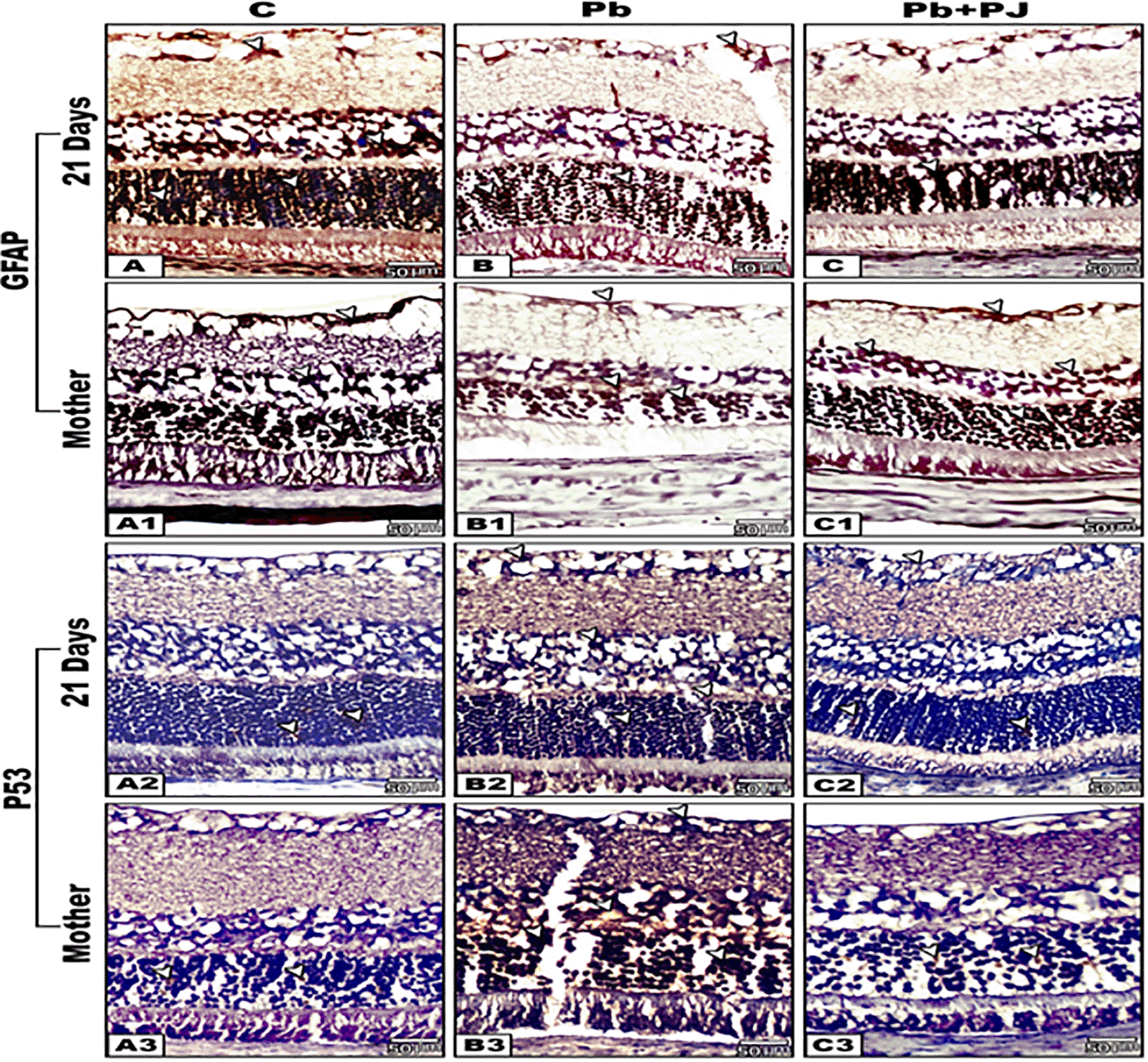
(The software used to edit the images and labels: Adobe photoshopCS8me).
The arrows heads indicate the degree of immunoreactivity of GFAP and P53.
Immunohistochemical reaction for GFAP
The immunoreactivity of GFAP appeared strong in the retinal sections of control neonates of rats if compared with their mothers. However, the retinal sections of Pb-exposed mother rats and their neonates displayed weak to moderate expression of GFAP if compared with control. Further observations revealed that the retinal sections of group 4 (Pb plus PJ) expressed a moderate to strong immunoreactivity for GFAP that was approximately similar to that of the control. For all studied groups the immunoreactivity of GFAP appeared prominent in ONL and INL rather than the other layers (Figure 6A-C1).
Immunohistochemical reaction for P53
The retinal sections of control mother rats and their offspring showed negative to weak immune expression for P53 protein. In contrast, an intense reaction for P53 was observed in all retinal cell layers of Pb-exposed mother rats and their neonates. In Pb-treated mother rats post-supplemented with PJ and their offspring, the retinal sections showed pronounced decrease in the degree of P53 immuno-reactivity (Figure 6A2-C3).
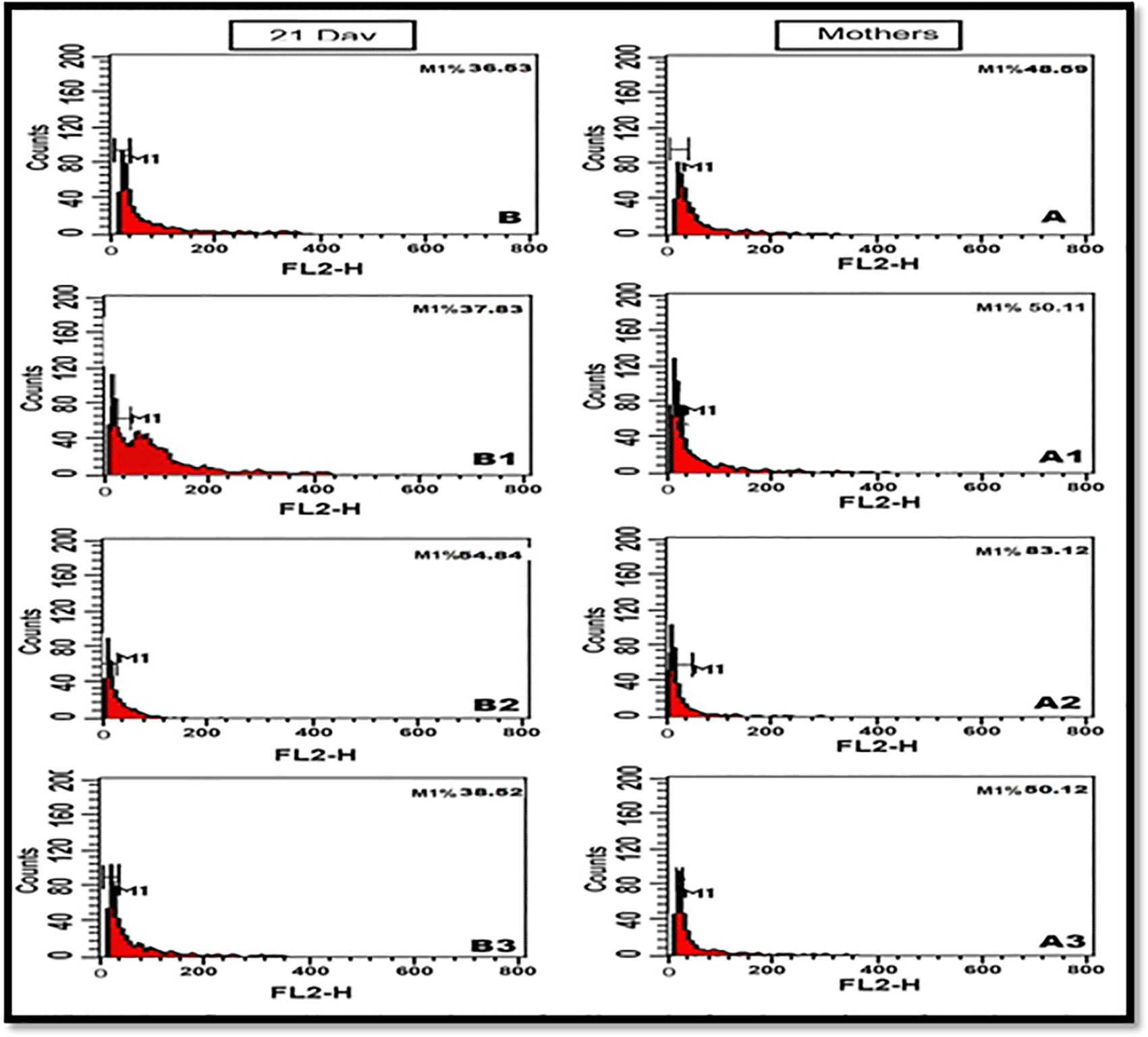
Note: highly % value of apoptotic cells in Pb-exposed groups of mothers rats and their offspring (83.12%, 50.12%) in comparing with control value (48.59%, 36.53%) respectively. Pomegranate juice successfully restored the mean % values of apoptotic cells near to the normal values.
The obtained flow cytometric results revealed that the mean percentage value of cellular apoptosis in the retina of Pb-treated mothers (83.12% versus 48.59%) and their offspring (54.84% versus 36.53%) was significantly different than that of control. Post-supplementation of PJ to Pb-treated group, the mean % values of apoptotic retinal cells were significantly decreased compared to the control values (50.12% for mother rats and 38.52% for offspring).
Lead exposure is known to disrupt most of body processes due to its toxicity to our vital organs, particularly our bones, heart, kidneys and nervous system.1,2,41 However, there has been little research into its direct effects on eye, a fundamentally cognitive process. Due to the direct correlation between the development of eyes and the central nervous system (CNS), there is no doubt that the potency of lead can prohibit the development of the CNS will inevitably cause effects on the eye. These effects are collectively termed “optic atrophy” or “blurred vision”, appearing only in cases of lead poisoning severe enough to cause brain damage.14,42 Pomegranate has a protective role as a phytotherapeutic agent due to its active ingredients like tannins,43 phenolic acids44 estrogenic flavonoids and conjugated fatty acids.45,46 Accordingly, this work was done for the first time to evaluate the possible ameliorative effects of pomegranate juice against lead acetate-induced retinal toxicity in pregnant rats and their neonates. Previous studies revealed that Pb compound can induce toxic effects on the mothers and their pups due to its transfer from the mothers to their pups during gestation and suckling periods.8
The result of the present work revealed that the mean body weights of Pb-exposed mothers and their offspring were significantly lowered than the control group. However, post supplementation of PJ to Pb-induced rats the body weight was successfully restored near to that of control. The obtained results are in agreement with previous researchers.47,48 Aprioku and Siminialayi49 reported that the decreased body weights of embryos maternally induced with Pb compound may be attributed to oxidative stress-mediated deleterious alteration in the ovarian and/or placental functions which interfere with fetal nutrition and O2 utilization. Additionally, placental disorders may also result in inhibition of transport of essential compounds to the fetuses, and consequently inhibit the normal growth rate of embryos. Another study explained that the toxic ions are one of the factors which decrease the body weight via the mechanism involving disturbance in metabolic enzymes.50 Restoration of body weight after supplementation of PJ to Pb exposed group of mother rats and their offspring may be attributed to the antioxidant constituents of PJ which allow easy assimilation of all the nutrients found in the blood stream by the body.51
Several histological lesions were recorded in the retinal cell layers of Pb-treated mothers’ rats and their offspring. Such alterations were represented by hypertrophied RPE, detached photoreceptors with fragmented outer segments and cytoplasmic vacuolation as well as the nuclei of the ONL and INL were aggregated in irregular clusters. Additionally, the GCL was highly vacuolated and lost its normal striation pattern. Fox and Chu52 reported that adult rats’ exposure to low-level Pb results in long-term selective photoreceptor deficits. Another study revealed that lead acetate or its derivatives could induce deleterious histological changes on the embryonic development of RPE and photoreceptors resulting in retinal detachment.53 Raafat et al.38 found that lead toxicity in mother rats could induce folding of pigment epithelium and fragmentation of photoreceptors outer segments. It had been postulated that the fragmentation of photoreceptors segments as well as appearing of cytoplasmic vacuolation under lead neurotoxicity is mainly due to rupture of bi-membranous discs and mitochondria.54 Elgohary et al.55 elucidated that aggregation of nuclei is an evident sign for pyknosis. Raafat et al.38 proved that animals that received lead acetate have swollen ganglion cell layer. The histopathological signs caused by lead acetate were more pronounced in the mothers and their offspring of 21 days old in comparing with the other two ages. Such variation in the degree of effects may be attributed to long period of exposure to lead acetate.
The results of the TEM study revealed that Pb led to the creation of deleterious ultrastructural changes all-over the retinal cell layers especially in the RPE and photoreceptor cell layers. In lead acetate treated mother rats and their offspring of 21 days old, the RPE cells displayed pyknotic nuclei, swollen and vacuolated mitochondria, vacuolated cytoplasm and remarkable disassembled Bruch’s membrane. Similar observations were recorded in the RPE of rabbit56 and rat neonates.57 Also similar alterations were noticed in the RPE of monkeys that were exposed to mercury54 and in humans exposed to cadmium.58,59 Vacuolation of the RPE cells post lead acetate-treatment may represent a type of cellular defense against its toxicities as well as a source of accumulating toxic agents interfering with its biological interactions in cell metabolism.60 Schlötzer-Schrehardt et al.61 reported that retinal detachment is mainly attributed to fragmentation of Bruch’s membrane.
Further observations by the TEM study revealed that the photoreceptors of lead acetate treated mothers and their offspring at 21 days old exhibited severe deleterious changes especially in mothers. Such changes were apparently represented by fragmented outer and inner segments with obvious vacuolation among them. Similar ultrastructural findings were recorded in retina of experimental animals after exposure to heavy metals.62,63 Furthermore, the previous experimental studies revealed that the low or moderate Pb dose could induce disorganization of photoreceptors in the mothers and children.64 Sanders et al.65 suggested that degeneration of photoreceptors under the influence of Pb is mainly due to the binding of lead and calcium to the internal metal-binding site of the mitochondrial transition pore, subsequently open the transition pore, and stimulate the cytochrome C-caspase activation cascade leading to death of photoreceptors. Moreover, the ONL and INL of lead acetate treated mothers and their offspring at 21 days old showed severe pyknotic nuclei, cytoplasmic lysis and vacuolation as well as remarkable wide intracellular spaces. Similar ultrastructural alterations were recorded in the two nuclear layers of adult rats66 and mice67 after exposure to low level of lead during gestation period.
GFAP is an intermediate filament protein that is expressed by numerous cell types of the central nervous system (CNS) including astrocytes68 and ependymal cells.69 GFAP is involved in many important CNS processes, including cell communication and the functioning of the blood brain barrier.70 Also, GFAP has been shown to play a role in mitosis by adjusting the filament network present in the cell.71 Our results revealed that the retinal sections from Pb treated mothers and their offspring exhibited weak expression for GFAP compared with the control group. The reactivity for such protein was more confined to the nerve cell fibers of ganglion layer, Muller cells and ONL. Similar observations were recorded in the brain of rat offspring72 and adult male rats post-treated with lead acetate.73 Chen et al.74 reported that depletion of GFAP activity plays a crucial role in activation of caspase-3 which would lead to increased apoptosis.
P53 is a tumor suppressor gene that in an inactivated form tends to be associated with a high risk of certain cancers and inhibition of apoptosis.75 Moreover, most evidence suggests that the key contribution of P53 to apoptosis is primarily dependent on transcriptional activity. P53 has the ability to activate transcription of various proapoptotic genes.76 Therefore, increased P53 activity can also trigger apoptosis by repression of antiapoptotic genes, such as surviving, thus promoting caspase activation.77 In compared with control, strong immunohistochemical reactivity for P53 protein was markedly observed all-over the retinal cell layers of Pb treated mothers and their offspring. According to the previous discussion, the decreased GFAP activity and overexpression of P53 reactivity in the retina of lead acetate groups confirmed an elevated risk of cellular apoptosis and that lead is the strongest neurodegenerative agent. Further examination to confirm the apoptosis was carried out by flow cytometric analysis to detect DNA damage. The results of the present work showed a significant increase in the percentage of cellular apoptosis in Pb treated groups in relative to control and pomegranate groups. Jia et al.78 revealed that lead acetate could induce a progressive loss in human mesangial cells viability together with a significant increase in the number of apoptotic cells using the assay of flow cytometry. Another study explained that Pb could induce oxidative stress, DNA damage and alteration of P53, Bax and Bcl-2 expressions in mice.79 The main mechanism of DNA damage caused by heavy metal ions, which contributed to genotoxicity, is the excess liberation of free radicals. These free radicals attack DNA double chains and break them. If the broken DNA strands cannot be repaired timely, it will affect the function of DNA and result in genotoxicity.80
In the present work, the pomegranate juice exerts ameliorative effects against the retinal histopathology as well as the immunohistochemical alterations induced by lead acetate. The pomegranate has been regarded as a “healing food” that plays a role in regulation of metabolism.80 It was commonly used as an anti-inflammatory, and in the treatment of ulcers, diarrhea, hemorrhages, microbial infections and respiratory diseases.32,81,82 Improvement of pomegranate juice against lead acetate treated retina may explain the reduction of inflammation, re-differentiation of retina and regulation of apoptosis as well as maintain normal pattern of GFAP and P53. All of these beneficial effects could be attributed to the polyphenols inclusion of pomegranate which has antioxidant capacity.
Detailed studies are required to understand the mechanism by which pomegranate juice can exert beneficial effects on the retina.
On the basis of our findings, the oral administration of PJ to lead acetate-treated rats during gestation and lactation could ameliorate the damage to retinal layers and restore their structure as well as enhance the antioxidative defense system due to presence of bioactive polyphenolic compounds which play a role in scavenging free radicals, and also prevent DNA damage.
figshare: Raw data for body weight. https://doi.org/10.6084/m9.figshare.19276061.v283
This project contains the raw data on rats’ body weighs.
figshare: Data of Flow cytometry. https://doi.org/10.6084/m9.figshare.19294352.v284
This project contains the flow cytometry data.
figshare: Underlying data.pdf. https://doi.org/10.6084/m9.figshare.19237473.v485
This project contains the images used in the manuscript.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The underlying image data for this study are too large to share openly. The image files are predominately JPG files, and approximately 200 MB (210,739,647 bytes) in size. Considering the large size and multiple images (n=280), the image files will be shared on request to readers. Please contact the corresponding author (beltagyaaa@yahoo.com) if you would like to request access to the image files. Representative images are shown in the figures and can be found in the Figshare repository.
figshare: ARRIVE checklist for ‘Evaluation of lead toxicity on the retina of pregnant rats and their pups: the possible ameliorative role of pomegranate juice’. https://doi.org/10.6084/m9.figshare.19237293.v3
The authors would like to thank the Light and Electron microscope unit of Mansoura University, for providing facilities to bring to this work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroprotection.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a retinal cell biologist with particular interest in retinal degenerations and neuroimmunology. Techniques include cellular (histology, cytometry) and molecular (RNA profiling) methods for interrogating changes in retinal pathophysiology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Apr 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)