Keywords
Orally disintegrating tablets, Solid lipid nanoparticles, ADMET properties, Centella asiatica; Parkinson's disease
This article is included in the Nanoscience & Nanotechnology gateway.
Orally disintegrating tablets, Solid lipid nanoparticles, ADMET properties, Centella asiatica; Parkinson's disease
Neurodegenerative disorders describe the progressive loss of structure or function of neurons, including neuronal cell death.1 Parkinson’s disease (PD) is the second most common chronic and progressive neurodegenerative disorder after Alzheimer’s disease (AD) that affects body movement, called progressive because the disease develops gradually and worsens over time. In addition, this disease also has a significant impact on several communities, both socially and economically for the sufferer.2 Clinical pathology and diagnosis of PD revealed loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the presence of Lewy bodies containing α-synuclein. The deficiency of dopaminergic neurons inhibits some motor functions, manifesting in tremor, rigidity, bradykinesia, and akinesia.3 PD therapy with a compensatory approach compensates for the effects of dopaminergic neuronal deficits targeting clinical and ongoing symptomatic treatment to manage PD, and levodopa is considered the gold standard for treating PD. In addition, alternatives are used to treat PD to slow or stop the progression of diseases monoamine oxidase-B (MAO-B)-inhibitors, Catechol-O-methyltransferase (COMT), dopamine antagonist, A2a antagonist, anticholinergics and glutamatergic.4 Current treatment strategies and recommendations in managing PD, including pharmacotherapy and supportive therapy, only relieve symptoms with serious side effects. While complementary and alternative medicine, including traditional medicine, is considered to have the ability to protect neurons and reduce side effects efficiently.5,6
Centella asiatica (L.) Urb. (CA) is a herbal medicinal plant with high product value.7 This plant which is commonly known in Indonesia as Pegagan has been used in traditional medicine, its ethnopharmacology applications are wide in various cultures and countries, besides its biological effects have been proven in multiple studies.8 The main chemical component of CA responsible for pharmacological activity is triterpene, consisting mainly of asiaticoside, asiatic acid, madecassoside, and madecassic acid.9 Several pharmacokinetic studies have confirmed that the bioactive compounds of CA provide neuroactive effects that have potential for use as neurotherapy. In a recent study by Hanapi et al. (2021), asiatic acid, asiaticoside, and madecassoside have high permeability and can cross the blood brain barrier (BBB).10 Bioactive components of CA also exert cognitive effects in aging and neurodegenerative diseases,11 where bioactive CA has been shown to protect against hippocampal dysfunction and improved cognitive performance in rat models.12 Neuroprotective effects have also been found in the bioactive component of CA in several disease models; CA extract was able to protect rotenone-induced parkinsonism rats against lipid peroxidation, dopaminergic neuronal death, and locomotor deficits13; CA stimulates nuclear factor erythroid 2–related factor 2 (Nrf2)-mediated antioxidant response and reduces oxidative stress contributing to improved neurologic health in AD model mice14; in addition, the antioxidant properties of asiaticoside were shown to increase the stability of the neurotransmitter dopamine and decrease α-synuclein aggregation in rotenone-induced PD zebrafish.15 CA has been shown to have anti-inflammatory effects; in a study by Qian et al. (2018) reported that asiatic acid effectively prevents lipopolysaccharide (LPS)-induced neuroinflammation in microglial cell line by increasing Sirtuin 1 (Sirt1) expression, attenuating inducible nitric oxide synthase (iNOS) expression and reducing inflammatory cytokine expression16; asiatic acid was shown to protect BV2 cells from LPS-induced damage by suppressing NLR family pyrin domain containing 3 (NLRP3) expression and ameliorating mitochondrial dysfunction.17 It is well known that triterpenoids from CA have beneficial effects on neurological and skin diseases, confirmed through clinical studies.18,19 However, the main compounds from CA, such as asiaticoside and madecassoside, show limited water solubility and usually have low absorption, so their oral bioavailability is low.9 Therefore, in this study, we evaluated absorption, distribution, metabolism, excretion, and toxicity (ADMET) using in silico research and developed a lipid-based nanocarrier as an ideal CA delivery system for oral administration, thereby increasing its bioavailability and effectiveness for the therapy of neurodegenerative diseases.
Parallel with developments in nanomedicine, lipid-based nanocarriers can be categorized based on their physicochemical properties and the manufacturing process consisting of liposomes, transferosomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs).19,20 SLNs is a lipid-based nanocarrier system that is widely explored for targeted drug delivery into the brain,21 with its relatively small particle size making it efficient and causing an increase in the surface area of insoluble drug particles, which in turn results in increased absorption through monolayer cells of the gastrointestinal (GI) tract.22 In addition, SLNs are an alternative in drug delivery systems with considerable advantages; they have good physical stability, increase the solubility and bioavailability of hydrophobic drugs23; high drug loading capacity of hydrophilic or lipophilic drugs24; protects sensitive active drug, and allows controlled drug release25; they are produced from physiological, biocompatible and biodegradable lipids with less or no biotoxicity making them safe and feasible for large-scale production at low cost.26 SLNs have lipophilic properties, and their small size makes them efficient in drug delivery to the central nervous system (CNS) by prolonging their circulation time in the blood and making them more effective across the BBB.27 In addition, SLNs are used as vehicles in delivering curcumin through the BBB to the brain, which is practical and potential for treating brain diseases, particularly AD and PD.28 SLNs have lipophilic features and appropriate size, so they are colloidal drug carriers easily penetrate the CNS.29 Therefore, drugs encapsulated in SLNs, which are further formulated in capsules or tablets, can be a successful attempt to reduce erratic absorption, increase bioavailability, and enhance lymphatic uptake.30
The most common oral formulations developed in the pharmaceutical industry are tablets or capsules because of their easy administration, flexibility, and compactness in manufacture.31 However, older adults, children, and patients with symptoms of dysphagia such as PD usually experience discomfort in drinking water that causes coughing and difficulty swallowing.32 Orally disintegrating tablets (ODTs) are described as solid dosage forms that have rapid disintegration and dissolution even in the presence of less saliva in the oral cavity and provide greater convenience and ease of use to improve adherence in elderly and geriatric patients. In addition, ODTs also have advantages such as good stability, easy manufacture and administration, fast drug absorption, and increased bioavailability.33,34 Direct compression is the most straightforward and most economical method of manufacturing ODTs where the active pharmaceutical ingredients (API) are only mixed with excipients which are then compressed into tablets. Despite the simplicity of the direct compression process, the excipient must meet some additional requirements as a particular product to be marketed as a global tablet, such as adequate flowability, compressibility, and compaction ability.35 A recent study by Zhang et al. (2020) reported that direct compression technology used in levodopa or benzylhydrazine ODTs had been successfully optimized with a relatively short disintegration time and fast dissolution profile, thus providing ease of use for Parkinson’s patients.32
The fact that nanoencapsulated CA-SLNs ODTs can be rapidly disintegrated in the mouth, further enhancing their absorption and bioavailability, makes it a good prospect for oral application, so the research is essential. This study aimed to develop a formulation of ODTs containing nanoencapsulated CA-SLNs with lipid-based nanocarriers as an oral delivery system, rapid disintegration, and dissolution, thereby providing greater convenience and ease of use to PD or dysphagia patients. ADMET prediction was used to evaluate the parameters of the physicochemical properties of CA compounds. Particle size, polydispersity index, zeta potential, and encapsulation efficiency of CA-SLNs were characterized to determine the physicochemical properties of the optimized lipid-based nanocarriers, and pre-compression properties of CA-SLNs powders such as flow time, angle of repose, bulk density, tap density, Hausner ratio, compressibility index, and moisture content were analyzed. Post-compression properties such as weight variation hardness, friability, wetting time, water absorption ratio, disintegration time, and content uniformity of the manufactured CA-SLNs ODTs were evaluated to obtain good quality tablets.
This study represents a development of CA-SLNs ODTs with several advantages that will be used as supportive therapy in Parkinson’s disease, and in this experiment did not involve live subjects (humans or animals), so the Ethical Committee, Faculty of Medicine, Universitas Brawijaya, Indonesia confirmed that no ethical approval was required. This experiment took place at the Pharmaceutical Science Laboratory, Faculty of Medicine, Universitas Brawijaya, from August 9 to December 20, 2021.
To predict the ADMET profiles of active compound CA such as asiatic acid (CID_119034), asiaticoside (CID_108062), madecassic acid (CID_73412), and madecassoside (CID_161823) were obtained from PubChem, and used the in silico method with the QikProp v6.4 module, Schrödinger, 2020-236 to determine the physicochemical parameters. In addition, several alternative open access in silico tools that can predict ADMET profiles and drug-likeness that academicians and industries most frequently use are ADMETlab, admetSAR, SwissADME, FAF-Drug, and TOPKAT.
Certified CA with 0.29% asiaticoside content obtained from UPT Material Medica Batu City, East Java, Indonesia. Briefly, fresh CA leaves were made into powder by washing, cutting, drying, and crushing. Furthermore, 100 g of CA powder was extracted by maceration method for 24 hours in 900 mL of 96% v/v ethanol. The CA solvent was evaporated at 45°C at low pressure using a rotary evaporator (Büchi Rotavapor R-300 System B-301, Switzerland). Then the filtrate was evaporated and maintained at -20°C. The CA extract obtained was stored in a dark and dry place until needed.
CA-SLNs were prepared by hot homogenization method. Briefly, stearic acid (50 g) was melted in a glass vial with a magnetic stirrer at 80°C. Then the CA extract was dissolved in liquid lipid and stirred until homogeneous, and formed a lipid phase. The aqueous phase consisting of tween 80 and PVP K-30 was dissolved in 200 mL of distilled water and brought to a temperature of 100°C. Keeping the respective temperatures, the lipid phase was added to the aqueous phase and homogenized with a high-performance homogenizer (Ultra-Turrax® T25, IKA, Germany) at 25,000 rpm for 5 minutes. Thus, a suspension of CA-SLNs was obtained, and the results were stored at room temperature. The CA-SLNs were then dried by adding aerosil at a ratio of 1:4 (1g CA-SLNs added 4g aerosil) and stirred to produce a powder.
The dynamic light scattering (DLS) technique by Malvern Zetasizer (Zetasizer Nano ZS-90, Version 7.01 Malvern Instruments Ltd, UK) assessed particle size distribution profiles. DLS measurements were used to determine the mean particle size, polydispersity index, and zeta potential at a fixed scattering angle of 90° at 25°C. CA-SLNs were suspended in distilled water and then vortexed. The dispersion was diluted with deionized water, and 1 mL of this sample was taken in a single-use plastic cuvette for measurement. The particle size distribution and polydispersity index were obtained from the ZetaSizer Nano software (ZetaSizer, Version 7.01, Malvern Panalytical, Malvern, UK), while the zeta potential was measured by electrophoretic light scattering. Analysis was performed in triplicate and presented as mean ± standard deviation.
The encapsulation efficiency (EE) and loading capacity (LC) were analyzed by ultrafiltration using a centrifugal ultrafiltration tube (Amicon® Ultra-4 10 kDa cut-off filter, Millipore, Billerica, MA, USA). Briefly, CA-SLNs were dissolved in sufficient quantities from phosphate buffer pH 6.8 and then filtered. An aliquot (1 mL) of CA-SLNs was placed in a centrifuge tube and centrifuged at 4000 rpm for 8 min at room temperature using a centrifuge (Centrifuge Z 327 K, Hermle Labortechnik GmbH, Wehingen, Germany). The concentration of CA-SLNs un-encapsulated in the filtrate was further determined by measuring the absorbance with ultraviolet (UV) spectrophotometry (UV-1601 spectrophotometer, Shimadzu Corporation, Kyoto, Japan) at 272.2 nm. Analysis was performed in triplicate and presented as mean ± standard deviation (SD). EE and LC are expressed in percentages calculated according to Equations 1 and 2;
where Wtotal is the total weight of CA in SLNs, Wfree is the weight of free CA in SLNs, and Wlipid is the lipid weight in SLNs.
CA-SLNs ODTs were prepared by the direct compression method. Croscarmellose is used as a superdisintegrant. The composition of the various formulations is shown in Table 1. All ingredients were sieved to produce 30 mesh separately, then weighed according to the required weight, and all except magnesium stearate were stirred homogeneously for 5 minutes. The mixture was then lubricated with magnesium stearate and mixed for 3 minutes. After that, the powder mixture was compressed into tablets using a single punch tablet machine (Tablet Press EP-1, Erweka, Heusenstamm, Germany), with a total weight of each tablet of 450 mg.37
Flow times, angle of repose, and moisture content
25 g of pre-compressed powder was put into Flodex Tester (Powder flowability tester Flodex™, Hanson Research, USA) to evaluate its flow times and angle of repose as well. The flow time was measured by dividing the sample mass by the time needed to flow through Flodex Tester orifice completely.37 The angle of repose was calculated using Equation 3;
where h is the height of mass powder the flow through Flodex Tester orifice, and r is the radius formed by mass powder.
25 g of pre-compressed powder was introduced into a moisture analyzer (MB23, Ohaus Corporation, Shanghai, China) to evaluate the moisture content.
Bulk and tap density, compressibility index, and Hausner ratios
100 mL Vb of pre-compressed powder was put into a graduated cylinder which was weighed.37 After the pre-compressed powder weight m (g) is determined, the bulk density ρb (g/cm3) is calculated using Equation 4;
After that, the pre-compressed powder was put into a 250 mL graduated cylinder, tapped gently until the powder volume stabilized, and the tapped volume Vt (mL) was recorded.37 The tap density ρt (g/cm3) was calculated using Equation 5;
The compressibility index and Hausner’s ratio were also calculated from bulk density and tap density to assess flowability using Equations (6) and (7), respectively.
Hardness test
The hardness test (newton, N; n=10) of randomly selected tablets from each formulation was tested using a tablet hardness tester (Tablet Hardness Tester HC6.2, Charles Ischi AG, Testing Technology, Switzerland). Results are presented as mean ± standard deviation.38
Weight variation
Overall, 20 individual tablets selected randomly from each formulation were weighed using an analytical balance of ±0.0002 g (Ohaus Pioneer PX224/E Analytical Balance, Ohaus Corporation, Parsippany, USA). Weight variation was evaluated by considering the standard deviation of tablet weight. Results are presented as mean ± standard deviation.39
Friability test
The friability test was carried out on 20 tablets selected randomly from each formulation, then weighed accurately (Wi) and put into the friability tester (AE-1 Friability + Abrasion Tester, Charles Ischi AG, Testing Technology, Switzerland), rotated at 25 rpm for 4 minutes. The tablets were de-dusted and weighed accurately (Wf).37 Friability (%) was calculated using Equation 8:
Wetting time, water absorption ratio, and disintegration time
The filter paper was folded twice and placed in a 6.5 cm diameter Petri dish containing 6 mL of distilled water with methylene blue. The tablet is placed on the paper, and the time taken for wetting is recorded t(s).37 Then the wet tablets were weighed again, and the water absorption ratio R (%) was calculated using Equation 9;
where m1 is mass of tablet before wetting (g), m2 is mass of tablet after wetting (g), and R is water absorption ratio (%).
The disintegration times tests were performed on six tablets selected at random from each formulation and determined in 750 mL distilled water at 37 ± 1°C using a disintegration tester (Erweka ZT301, Heusenstamm, Germany). The tablet was considered completely disintegrated when all the particles passed through the screen.37 The disintegration time of individual tablets was recorded, and the mean ± standard deviation was reported.
Drug content uniformity
The content uniformity test was carried out on ten tablets selected at random from each formulation and weighed, then suspended in 100 mL of a mixture of methanol: ultrapure water (50:50, v/v). Each sample was filtered through a PVDF filter and analyzed in triplicate (n=3) using a UV-Vis Spectrophotometer (UV-1800, Shimadzu Corporation, Kyoto, Japan) at 291 nm. The acceptance value (VA) was calculated using Equation 10;
where M is the label claim (%), X is the measured content of CA or CA-SLNs, k is the acceptability constant (2.4), and s is the standard deviation.39
Dissolution study
The in vitro dissolution test on six tablets was randomly selected using the USP dissolution apparatus II (Erweka DT 708, Heusenstamm, Germany). The tablet assay was carried out in 900 mL of phosphate buffer pH 6.8 adjusted at 37 ± 0.5°C (pH, 6.8 ± 0.05), with the paddle rotated at 50 rpm for 45 minutes. Each sample of dissolution media was taken 3 mL with predetermined time intervals (2, 5, 10, 15, 30, 45, and 60 minutes) and filtered using a PVDF filter. The samples were analyzed in triplicate (n=3) using a UV-Vis Spectrophotometer (UV-1800, Shimadzu Corporation, Kyoto, Japan) at 291 nm.40
Results are presented as mean ± standard deviation (SD). The results were statistically analyzed with Mann-Whitney U test using SPSS software (SPSS, Version 21.0, IBM Inc. USA, RRID:SCR_016479). When p value was less than 0.05, the difference between the results was considered significant.
To improve the quality control of the active compound as a drug and explain its ability to reach the target protein, we predicted the ADMET of the active compound CA by in silico method. Computational approaches are beneficial in drug design to reduce costs and time and minimize failure in the clinical stage. This study used QikProp v6.4 module of Schrödinger, 2020-2 to predict ADMET and physicochemical properties of active compounds CA (asiatic acid, asiaticoside, madecasic acid, and madecasoside). Jorgensen’s “Rule of Three” and Lipinski’s “Rule of Five” predict the physical properties, relevant properties, and similar features of drugs as pharmaceutical products. The expected parameters and recommended values are summarized in Table 2, which shows that all parameters are generally within the standard range and slightly violate the abovementioned rules.41
| Predictor | Chemical compounds | |||
|---|---|---|---|---|
| Asiatic acid | Asiaticoside | Madecassic acid | Madecassoside | |
| mol_MW | 488.706 | 959.133* | 504.706 | 975.132* |
| dipole† | 3.541 | 3.768 | 3.775 | 3.735 |
| volume | 1426.390 | 2460.933 | 1436.048 | 2562.618 |
| PSA | 103.508 | 290.824* | 119.784 | 309.139* |
| donorHB | 4.000 | 12.000* | 5.000 | 13.000* |
| accptHB | 7.100 | 30.900* | 8.800 | 32.600* |
| QPlogPo/w | 4.117 | 48.142* | 3.250 | -2.080* |
| QPlogS | -5.407 | -1.350 | -4.683 | -2.686 |
| QPlogBB | -1.325 | -4.263* | -1.547 | -5.260* |
| #metab‡ | 5 | 14* | 6 | 15* |
| CNS | – | – | – | – |
| QPPCaco | 47 | 4 | 29 | 1 |
| QPPMDCK | 23M | 1M | 13M | 0M |
| RuleOfFive | 0 | 3 | 1 | 3 |
| RuleOfThre | 0 | 2 | 0 | 2 |
| HumanOralAbsorption | 81 | 0 | 59 | 0 |
The physicochemical properties of CA compounds nanoencapsulated in SLNs using the hot homogenization method are summarized in Table 3. The prepared nanoencapsulated CA-SLNs with lipid-based nanocarriers showed a relatively uniform mean particle size distribution in the range of 37.91 ± 1.55 nm to 54.83 ± 1.40 nm with a polydispersity index ranging from between 0.14 ± 0.01 to 0.19 ± 0.01, the low polydispersity index value < 0.2 indicates that the particle size distribution is narrow and homogeneous with low variability from all formulations. Moreover, the negative zeta potential values ranged from -12.72 ± 0.69 mV to -10.27 ± 1.37 mV, indicating that the developed SLNs were stably dispersed without a tendency to aggregate. EE and LC were analyzed to evaluate whether CA can achieve high active loads and determine the amount incorporated in the lipid nanoparticles. All formulations were characterized by EE and LC ranging from 90.59 ± 1.63% to 95.07 ± 1.14%, and 3.99 ± 0.06% to 4.13 ± 0.06%, respectively. The very high EE values of all formulations indicated that functionalization did not affect the ability to incorporate CA in lipid nanoparticles efficiently. Overall, these results indicate that the CA-SLNs pre-formulation process used in this work is effective.
The physical pre-compression properties of the prepared ODTs powders (CA extracts and CA-SLNs) were evaluated to determine the bulk and tap density, powder flow (flow through an orifice, angle of repose, compressibility index, Hausner ratio), and moisture content as suggested by the Ph. Eur. 10th.37 The flowability of powders is an important property, especially in manufacturing tablets by direct compression method. As displayed in Table 4, all the optimized pre-compressed formulations of CA-SLNs and CA-extract powders showed good to excellent flow times; the results were found in the range of 4.56 ± 0.21 s and 5.05 ± 0.22 s. These results indicate good flowability properties of the powder to be formulated by direct compression method. An angle of repose analysis is carried out to evaluate the frictional force or cohesion between particles, and this test is the simplest in powder pre-compression. Analysis of the angle of repose for all formulations (CA-SLNs and CA extracts) of the optimized pre-compressed powder, the results were found in the range of 25.16 ± 1.47° and 29.16 ± 1.94°, which further supports the good flowability of all mixture. Powders with an angle of repose values in the range of 25-30° exhibit excellent flowability properties.42
The bulk density and tap density were evaluated to determine the rheological properties of the powder because the preparation, treatment, and storage processes will affect it. The data on bulk density and tap density obtained are very similar ranging from 0.53 ± 0.01 to 0.57 ± 0.01 g/cm3 and 0.65 ± 0.01 to 0.68 ± 0.01 g/cm3, respectively (Table 4). These results indicate that the pre-compressed powders have the same flowability properties. In addition, the compressibility index and the Hausner ratio method were used to predict the flowability characteristics of the powder. The compressibility index measures the stability and bridge strength of the powder, and the Hausner ratio measures the friction between particles, which is used in evaluating the flowability characteristics of the powder. The results showed that the compressibility index value ranged from 8.33 ± 1.50% to 11.83 ± 1.47%, and the Hausner ratio from 1.15 ± 0.01 to 1.17 ± 0.01 (Table 4). Based on the flowability scale in Ph. Eur. 10th, powders with a compressibility index of 1-10% and a Hausner ratio of 1.00-1.11 are considered excellent flow properties.42 This information confirms that the formulation of the optimized pre-compressed powder has excellent flow properties. Besides these values, in the manufacture of quality tablets, the moisture content must also be evaluated and controlled because the moisture content affects the flowability of the powder, when the moisture content is low, then the powder will flow better, in this study, the moisture content ranged from 2.58 ± 0.26% to 3.53 ± 0.32% which further supports good flowability properties.
The physical characteristics of powder pre-compression (CA-extracts and CA-SLNs) are presented in Table 4, indicating that the powder pre-compression has excellent flowability properties and is suitable for the direct compression method.
As displayed in Table 5, the physical characteristics of the ODTs formulation developed by direct compression method showed that the weight variation, hardness, friability, wetting time, water absorption ratio, content uniformity, and disintegration time all fulfilled the Ph. Eur. 10th specifications.37 Weight variation is one of the critical variables to ensure drug content uniformity. Table 5 shows the mean tablet weight and standard deviation for all formulations. No statistically significant differences in weight variation values for CA-extracted ODTs and CA-SLNs ODTs were noticed (Table 5, Mann-Whitney U test, p > 0.05), where CA-extract ODTs (F1 is 448.95 ± 1.93 mg), and CA-SLNs ODTs (F2, F3, and F4 are 449.35 ± 1.84 mg, 449.85 ± 1.98 mg, 449.55 ± 1.87 mg, respectively), this shows that the weight variation and standard deviation value in a tablet of less than 2.0 is acceptable with USP 43-NF 38 criteria.39 These results indicate that the obtained ODTs conform to the limits of both weight uniformity and tablet units with a very low coefficient of variation.
Because tablets prevent the risk of abrasion during packaging, handling, and transportation before use, they must possess optimal mechanical strength. The average hardness of tablet formulations developed by direct compression method is shown in Table 5. There is a significant difference between the hardness values obtained for CA-extracted ODTs and CA-SLNs ODTs (p<0.05). For CA-extracted ODTs it was found to be 24.01 ± 1.43%, and CA-SLNs ODTs (F2, F3 and F4 were 25.97 ± 1.84%, 29.17 ± 1.62 and 26.99 ± 1.54, respectively). According to USP 43-NF 38 (2021), the hardness test of the ODTs should be ≤ 30 N to maintain packaging, handling, and transportation.38 According to this information, the ODTs we developed showed a hardness value of <30 N (Table 5) and had good mechanical strength. They could withstand physical and mechanical stress conditions, also according to the properties of ODTs that rapidly disintegrate in the mouth. Another tablet property related to mechanical strength is friability. As shown in Table 5, all the prepared tablet formulations passed the Ph. Eur. 10th limit test where friability should be <1%.37 The average friability percentage of all tablet formulations (CA-extract ODTs and CA-SLNs ODTs) optimized by direct compression method ranged from 0.56 ± 0.02% to 0.89 ± 0.03%. Friability is related to tablet hardness, as shown in this study, the friability decreased, followed by increasing tablet hardness (Table 5). These results indicate that the developed ODTs formulation has stable mechanical strength during the manufacturing, packaging, and shipping processes.
Wetting time is strongly influenced by the composition, structure of the tablet formulation, swelling and wicking effect of the superdisintegrant. Wetting time values of all ODTs formulations are shown in Table 5; there is a significant difference between CA-extracted ODTs and CA-SLNs ODTs (Mann-Whitney U test, p > 0.05), where CA-SLNs ODTs formulations showed wetting time or methylene blue diffusion was approximately (F2, F3, and F4 were 25.66 ± 1.36s, 22.16 ± 1.94s, and 24.33 ± 1.03s, respectively) significantly lower than the CA-extracted ODTs formulations (F1was 27.66 ± 1.03s). This performance will further affect the water absorption ratios of the tablet and the drug release profile. Evaluation of the water absorption ratio found that the CA-SLNs ODTs formulation (F2, F3, and F4 were 58.50 ± 1.87s, 54.50 ± 1.37s, 56.16 ± 1.47s) were significantly lower than the CA-extracted ODTs formulations (F1 was 62.33 ± 1.21s; p < 0.05; Table 5). Wetting time and water absorption ratio are essential parameters for evaluating disintegrant expansion capacity in the presence of small amounts of water. In addition, disintegration time is a critical parameter for determining the optimal formulation of ODTs and distinguishes conventional tablets from ODTs with relatively fast disintegration time. In this experiment, we found that the disintegration time of the CA-SLNs ODTs formulation (15.66 ± 1.36s for F2, 14.50 ± 1.04s for F3, and 15.16 ± 1.16s for F4) was shorter than that of the CA-extracted ODTs formulations (17.33 ± 1.21s for F1) (Table 5). There is a significant difference between the in vitro disintegration times of CA-extracted ODTs and CA-SLNs ODTs (p<0.05). The disintegration time of ODTs set by the FDA (2008) is the 30s, whereas according to the Ph. Eur. 10th, the disintegration time is 180s longer.42,43 This confirms that the ODTs we have developed fulfil the requirements of the FDA and the Ph. Eur. 10th.
The content uniformity test is a parameter to ensure the homogeneity of the active substance of each unit of the drug dosage form and determine whether the content of each unit is within the specified limits. As shown in Table 5, the content uniformity test of ODTs formulation indicated that the AV value was < 15% for the ten individual unit criteria in the first stage, L1 (USP 43-NF 38).39 The CA-extracted ODTs formulations (F1 was 4.9%), and CA-SLNs ODTs formulation (F2, F3 and F4 were 4.1%, 3.2%, and 3.7%, respectively). In addition, the drug content for all formulations of ODTs developed were within USP 43-NF 38 limits (85-115%),39 where the label claims for CA-extracted ODTs formulations (96.45 ± 1.18% for F1) and CA-SLNs ODTs formulation (97.10 ± 1.13% for F2, 97.98 ± 1.12% for F3, and 97.44 ± 1.11% for F4).
ODTs are required for fast-acting dissolution, and the loaded drug must be released within a short period. In this study, phosphate buffer (pH 6.8) was used as a medium to simulate ODTs in the local environment. The in vitro dissolution profiles of the ODTs formulations are illustrated in Figure 1, They show that the percentage of CA-SLNs dissolved reaches 90% within 5 minutes in phosphate buffer of the CA-SLNs ODTs formulation. (F2, F3, and F4 which dissolved 92.36 ± 0.83%, 95.13 ± 0.35% and 94.06 ± 0.85%, respectively). Moreover, tablet dissolution efficiency was almost 100% within 10 minutes on the CA-SLNs ODTs formulations (F2, F3, and F4 which dissolved 96.03 ± 0.30%, 99.86 ± 0.20% and 98.66 ± 0.64%, respectively). These results indicate that all the prepared tablets fulfil the FDA and USP 43-NF 38 requirements for orally disintegrating tablets and allowed more than 85% of the API to be dissolved in 30 minutes.40,44
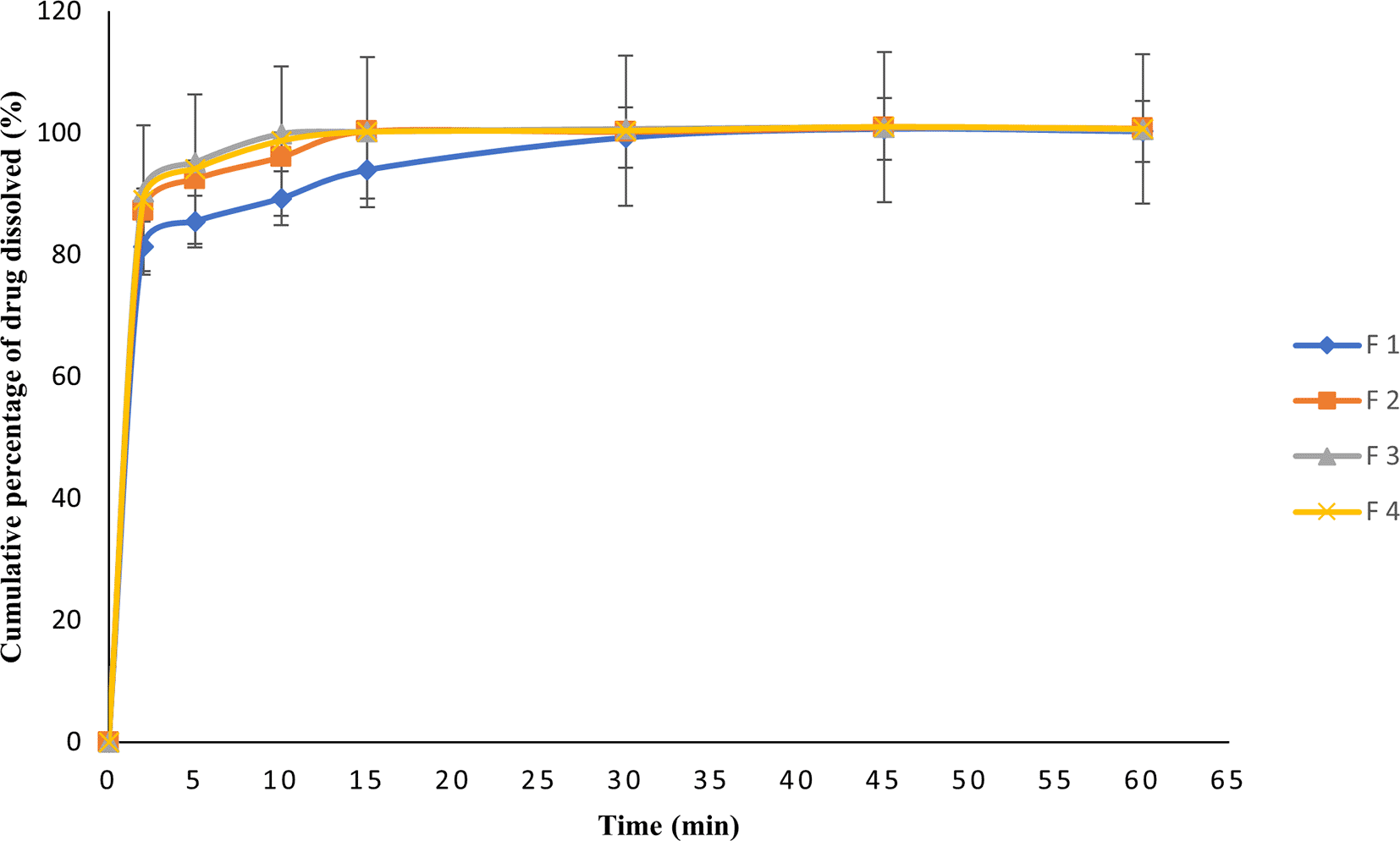
In vitro dissolution profiles of orally disintegrating tablets (ODTs) formulation containing Centella asiatica (CA)-extracts, and CA encapsulated solid lipid nanoparticles (CA-SLNs) in phosphate buffer (pH 6.8) at 37°C with a rotation speed of 50 rpm in a dissolution apparatus II. F1 is 200 mg/tablet CA-extract; F2 is 150 mg/tablet CA-SLNs; F3 is 200 mg/tablet CA-SLNs; and F4 is 250 mg/tablet CA-SLNs. Bars indicate standard deviation, n=3.
CA has emerged as a promising natural compound with biological properties. In this work, we are interested in its neurotherapeutic effects. However, the main compounds of CA, such as asiaticoside and madecassoside, are known to have limited water solubility and usually have low absorption.9 Several approaches have been applied to overcome this limitation, namely lipid-based nanocarriers that potentially and effectively protect active compounds from degradation in the bloodstream, enhancing oral delivery.45–47 This study was designed to develop a formulation of ODTs having rapid disintegration and dissolution, thereby providing greater convenience and ease of use to patients with PD with difficulty swallowing, nanoencapsulated CA-SLNs with lipid-based nanocarriers as an effective and potential CA delivery system through the BBB to the brain. ADMET prediction method is needed to develop active compounds as drug candidates and select potential therapies for further processing. A significant failure rate at the development stage of the active compound as a potential drug candidate was associated with ADMET deficiency; therefore, the computational method remains a reliable, cost-effective, and time-saving technique.48 The “Rule of Five” by Lipinski and the “Rule of Three” by Jorgensen were used to predict and evaluate specific molecules of compounds, such as physicochemical properties, pharmacokinetics, lipophilicity, solubility, and drug likeliness.49,50 According to the results presented in Table 2, all parameters were within the standard range, and the compound did not cause more than one violation of the “Rule of Five” (MW < 500, logP < 5, DHB ≤ 5, AHB ≤ 10, Positive PSA value) and “Rule of Three” (logS > -5.7, PCaco > 22 nm/s, PM < 7). In addition, the permeability of BBB plays a role in the early discovery of neurologic drugs due to its high-throughput.51 The log BB values, which guide compounds crossing BBB, were in the range of -5.260 to -1.325. Therefore, it can be said that the active compound CA is a potential drug candidate in the CNS because it can pass through the BBB. Most of the drugs are given orally to increase their effectiveness and pharmacokinetics, so they must be absorbed in the bloodstream through oral absorption in the gastrointestinal tract to be accessible to the brain, and Caco2 is used as a parameter in predicting intestinal permeability of drug candidates.52 Besides, the aqueous solubility (logS) is an important physicochemical property of compounds in drug discovery, and our study is still within the range of values determined. Based on the prediction findings of ADMET parameters, the active compound CA has good and promising pharmacokinetic, permeability, solubility, and toxicological properties, which may be suitable for clinical use.
Particle size and polydispersity index are essential characteristics of nanoparticles used to evaluate particle distribution, stability, and preliminary prediction of biological performance. Lipid-based nanocarriers generally have diameters between 10 nm to 1000 nm, and nanocarriers with sizes less than 100 nm allow increased permeability through the BBB and absorption in the brain.53 In this study, the average particle size distribution of nanoencapsulated CA-SLNs was lower than 100 nm. In addition, the polydispersity index of the nanoencapsulated CA-SLNs is precisely less than 0.2. This polydispersity index value measures the uniformity of the particle size distribution. SLNs formulations with polydispersity index values in the range of 0.0-0.5 were described as homogeneous and monodisperse; however, SLNs with polydispersity index values greater than 0.5 were described as heterogeneous and polydispersity.54 Zeta potential is an important parameter for predicting the tendency of particles to aggregate, the size of the surface electric charge of the particles, and provides information regarding particle stability.20 SLNs can be considered stable dispersion without agglomeration when the absolute value of negative zeta potential is around -30 mV.55 This study showed that CA-SLNs encapsulated were negatively charged, with a zeta potential value between +30 mV and -30 mV, indicating that CA-SLNs have high stability and low aggregation probability.
EE and LC are two important parameters to determine the potential application of SLNs as drug delivery systems that should be considered during the formulation development phase, where EE is described as the percentage of active ingredients encapsulated in nanoparticles, referring to the total active ingredient added for the preparation of SLNs (values range from 80–99%), while LC defined as the percentage of active ingredient encapsulated in nanoparticles referring to the total weight of the lipid phase (values range from 1–20%).56 Therefore, EE of CA-SLNs (95.07%) suggested that part of the hydrophobic active ingredients was lost during preparation of lipid-based nanocarriers by hot homogenization method. This minimal loss of active ingredients may be due to the organic solvent used to obtain the water-in-oil emulsion during the formulation process, which can extract the active ingredients from the lipid matrix of SLNs. In addition, the difference in the structure of the solid lipids used, where the dense lipids form fewer perfect crystals, causes an increase in the space in the dense lipid matrix to accommodate the active ingredients. The incorporation of CA into SLNs did not cause any loss of active ingredients during the formulation process, as it confirmed that CA-SLNs showed an EE value close to 100%. This evidence highlights the advantages of our lipid-based nanocarrier in obtaining high incorporation, given that CA is successfully incorporated into SLNs, with smaller particle sizes having higher EE and LC.
The direct compression method is one of the methods used to manufacture tablets without a granulation process. It requires appropriate additional ingredients, with several advantages such as being faster, simpler, lower production costs, and more accessible than other methods (wet and dry granulation), and providing integrity high mechanical properties of tablets.57 However, there are some conditions in using this method, such as the flowability properties and compressibility of the powder mixture must be suitable. Therefore, a pre-compression study was carried out to determine the flowability properties and compressibility of the powder mixture used in ODTs, which included the flow time, angle of repose, bulk density, tap density, Hausner ratio, compressibility index, and moisture content.58 As shown in Table 4, all the pre-compression parameters showed promising results to be developed in ODTs. The direct compression method was suitable for tablet preparation as suggested by the Ph. Eur. 10th 37. The angle of repose indicates friction or cohesion force between particles in powder; when angle of repose is lower than 30° for powder, they represent free-flowing behavior. The flowability properties and compressibility trend are described by Hausner’s ratio and compressibility index, respectively. Powder mixtures with compressibility index values in the range 1-10% and Hausner ratio values in the range 1.00-1.11 showed better flowability characteristics.37 So, when the value of the Hausner ratio is lower than 1.15 and the compressibility index value is less than 8.33%, the flowability properties are excellent. The compressibility index is an indirect measure of surface area, powder particle shape, bulk density, moisture content, and powder compactness.42 Since the bulk density is directly related to the particle size of the powder and tends to adhesion, it is vital in selecting packing materials and considerations of compression transport. In addition, powder flowability properties are also influenced by electrostatic interactions, moisture content, density, particle size, and shape.
All formulations developed with the direct compression method produce elegant and successful ODTs. All physical characterization tests are within the limits of the Pharmacopoeia. It is well known that individual dose unit weight variations guarantee a product’s dose uniformity. This parameter is crucial in giving the same dose of active substance during the therapeutic period to ensure the repeatable pro-health effect. As presented in Table 5; all analyzed ODTs passed the pharmacopoeial weight change test >90% of the tested tablets, and these results showed weight uniformity according to specifications (USP 43-NF 38).39 The most critical parameter of ODTs are friability, hardness, and wetting time. Those parameters directly influence the efficacy of the tablet. Tablet friability and hardness contribute to physical resistance and the ability to disintegrate in mouth. The best ODTs should have low friability and good hardness to prevent mass loss from tablet surface when being handled. On the other hand, the presence of crosscarmelose as the superdisintegrant may worsen the tablet hardness and its friability. At the same time, we need the crosscarmelose to obtain rapid disintegration within the mouth by only the presence of a minimal volume of saliva. As seen in Table 5, those critical parameters showed promising results for ODTs.
Determination of the content uniformity of active ingredients in tablet preparations is essential for quality control and is expressed as a percent on label claims. In this study, the test was carried out using a UV-Vis Spectrophotometer, which allowed the quantification of CA-SLNs and measuring their content in ODTs. USP 43-NF 38 recommends a limit of variation of 85-115%, from the content of all formulations of ODTs (CA-extracts and CA-SLNs) fulfil the criteria stated as 96.45% to 97.98% (Table 5).39 In addition, each tablet unit must have several active ingredients other than the amount stated in the content value and dosage uniformity to ensure the delivery of the correct dose of the active compound. This parameter is measured by AV calculation with a maximum value of 15%, and the results of our study showed that all formulations of ODTs (CA extracts and CA-SLNs) met these criteria (USP 43-NF 38).39 In vitro dissolution test is crucial for determining the release profile of CA-SLNs formulated in ODTs and to characterize the biopharmaceutical qualities in solid oral dosage forms, thus enabling control of the formulation quality. This test was carried out following USP 43-NF 38 conditions for tablet dissolution using apparatus II, with a dissolution medium of phosphate buffer pH 6.8. As shown in Figure 1 the release profile of CA-SLNs ODTs from different formulations. All formulations of CA-SLNs ODTs had similar dissolution profiles. Approximately 50% of CA-SLNs are released in 2 min and complete drug release is achieved in 15 min for F2, F3 and F4. Therefore, the cumulative release of the CA-SLNs ODTS prepared by direct compression method in this study is good and acceptable on the basis of the FDA and USP 43-NF 38.40,44
This study demonstrated that orally disintegrating tablets (ODTs) containing Centella asiatica (CA) encapsulated solid lipid nanoparticles (SLNs) were successfully developed with optimized formulations using direct compression method. ADMET study confirmed good oral bioavailability and safety of these compounds. In addition, CA-SLNs have been successfully optimized as an alternative delivery system to protect the active compound and increase its permeability through the BBB, allowing CA compounds delivery to the brain. Furthermore, CA-SLNs ODTs have been successfully developed with excellent flowability properties, relatively short disintegration time, fast dissolution profile, and fulfilled pharmacopoeial requirements. These results confirm that CA-SLNs ODTs may help manage PD, older adults, and pediatric patients with difficulty swallowing.
Figshare: Supplementary Data – Development and optimization of orally disintegrating tablets containing Centella asiatica solid lipid nanoparticles for supportive therapies of Parkinson’s disease, https://doi.org/10.6084/m9.figshare.19592935.v1.41
This project contains the following underlying data:
• Supporting information predicted ADMET properties of CA compounds.docx
• Raw data physicochemical characteristics of CA-SLNs.xlsx
• Raw data pre-compression properties of powder.xlsx
• Raw data physical properties of ODTs.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience, Parkinson's disease, molecular biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 13 May 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)