Keywords
Allergic rhinitis, ClinicalTrials.gov, Completeness, Informativeness, Trial Registration Data Set
Allergic rhinitis, ClinicalTrials.gov, Completeness, Informativeness, Trial Registration Data Set
AE: Adverse Event
AR: Allergic rhinitis
CONSORT: Consolidated Standards of Reporting Trials
FDAAA: The Food and Drug Administration Amendment Act
ICMJE: International Committee of Medical Journal Editors
MeSH: Medical Subject Headings
NCT: National Clinical Trial
OAE: Other Adverse Event
RCT: Randomized controlled trials
SAE: Serious Adverse Event
STROBE: Strengthening the Reporting of Observational Studies in Epidemiology
TRDS: Trial Registration Data Set
U.S.: United States
WHO: World Health Organization
Allergic rhinitis (AR) is a global health problem with a 10% to 30% prevalence in adults and up to 40% in children.1,2 The total cost of AR in the U.S. is estimated at $9 billion annually through direct health expenditures and lost productivity due to absence from work and school.3,4 Studies that test interventions, drug efficacy or behavioral factors that may stop the increase in prevalence or reduce the costs of AR are imperative. Therefore, data from such studies should be complete and consistent throughout multiple sources to ensure accurate evidence-based information that may be used by patients, clinicians, researchers, and professional organizations for decisions regarding therapy, establishing guidelines, or other.5–9 The accuracy and completeness of data from studies can be assessed from the presence of standardized data elements registered in a public registry such as ClinicalTrials.gov.5,10
For adequate trial registration and a precondition for publication in International Committee of Medical Journal Editors (ICMJE) member journals, the World Health Organization (WHO) and ICMJE, since 2005, require the completion of WHO Trial Registration Data Set (TRDS) items for basic, mandatory trial information.11–14 Two years later, on 9/27/2007, Section 801 of the Food and Drug Administration Amendment Act (FDAAA 801) went into effect and mandated the registration of clinical trials and reporting of basic results from them within 1 year of trial completion.15 Furthermore, the Final Rule of Clinical Trials Registration and Results Information Submission, implemented in January 2017, clarified which trials the FDAAA covers as well as requirements concerning registration and results submission.16,17 Additionally, in November 2017, WHO TRDS has been expanded with 4 new items and currently contains 24 items.18 As a result, the registration of trials in ClinicalTrials.gov as well as the consistency of information with corresponding publications has improved; however, discrepancies remain between trial information and results reported in ClinicalTrials.gov and corresponding publications more than a decade since the implementation of the FDAAA 801.19,20
Thus, we aimed to assess completed randomized controlled trials (RCTs) on AR registered in ClinicalTrials.gov for completeness, informativeness and major changes to WHO TRDS items as well as completeness of results data in ClinicalTrials.gov and corresponding publications.
All methods were performed in accordance with the relevant guidelines and regulations and there was no need for approval from an institutional review board (Ethics Committee of the University of Split School of Medicine), as this study is cross-sectional and historical cohort database study. We neither collected patient data nor performed experimental procedures.
Furthermore, all data used in this study are publicly available in the ClinicalTrials.gov registry and therefore no permission was required to access the data.
This study neither studied nor analyzed the raw data of other studies. We analyzed their data which were publicly registered in ClinicalTrials.gov and published in publicly available scientific journals. So no permission was needed here either.
Data from other studies registered in the ClinicalTrials.gov registry, which we analyzed, were anonymized by their authors, so no anonymization was required on our study.
Figure 1 shows our retrospective analysis. The start date (9/27/2009) of the search allowed at least two years for the study to post in ClinicalTrials.gov and publish results in journals. The last search date (10/4/2019) allowed more than 10 years from first to last posting and publishing of results.
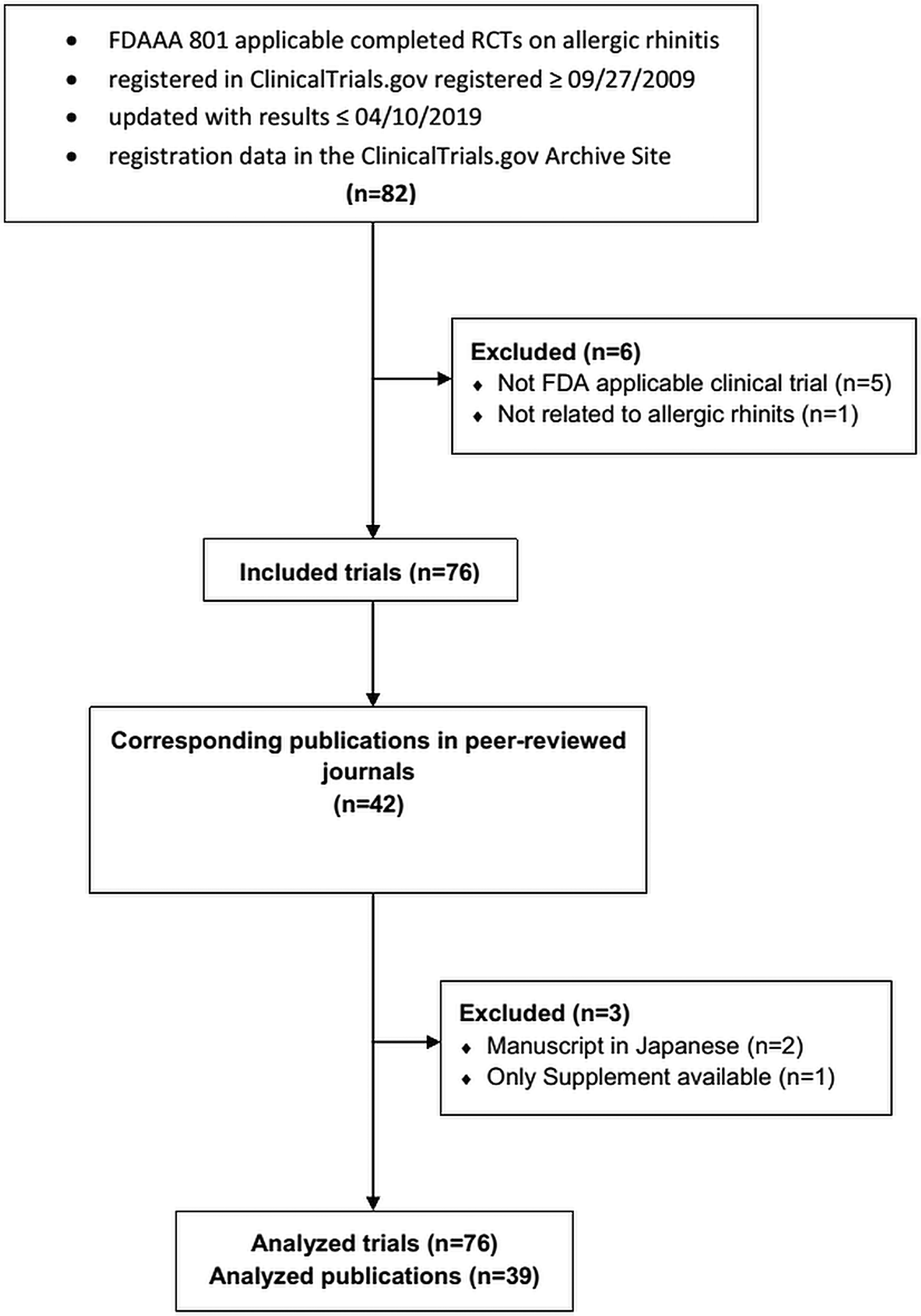
Flow diagram of retrospective cross-sectional study with inclusion and exclusion criteria. FDAAA: The Food and Drug Administration Amendment Act, FDA: The Food and Drug Administration.
We accessed the history of all changes in registration elements through the ClinicalTrials.gov Archive site. We searched ClinicalTrials.gov for completed RCTs using the following keywords: “allergic rhinitis”, “nasal allergies”, “rhinoconjunctivitis”, “hay fever”, and “atopic rhinitis”. We did not use Medical Subject Headings (MeSH) terms.
For trials with publications provided on ClinicalTrials.gov, we selected only those that reported the results of the current trial to find corresponding publications to our sample of AR RCTs. For trials without publications provided, we searched PubMed, Web of Science, Scopus, and Google Scholar with the National Clinical Trial (NCT) identifier provided by ClinicalTrials.gov in the trial record that is usually listed in the abstract or main text of published articles.21 If the initial search failed, we searched using the principal investigator's name and study title. Only full publications were compared with registered data. Publication dates were determined from online publication dates for articles made available ahead of print.22
Inclusion criteria are shown in Figure 1. Requirements for an applicable clinical trial according to the FDAAA 801 were verified according to the “Elaboration of definitions of responsible party and applicable clinical trial (ACT)” from 3/9/2009 for trials initiated after 9/27/2007 and according to the “Checklist for evaluating whether a clinical trial or study is an ACT” for those initiated after 1/18/2017.23
Differences between first and last registration were assessed from 20 out of 24 extracted WHO TRDS items, while we assessed differences between last registration and publications from 15 out of 24 items. The “Contact for Public Queries” and “Contact for Scientific Queries” items were excluded from both analyzes due to their unavailability in ClinicalTrials.gov while “Recruitment Status” item was excluded due to it's transient nature. Furthermore, the “Date of Registration in Primary Registry”, “Secondary Identifying Numbers”, “Secondary Sponsor(s)” and “Public Title” items were excluded from the analysis of the differences between last registration and publications due to their exclusion from journals. The “IPD Sharing Statement” and “Ethics review” items added to the WHO TRDS in November 2017 were not included in the analysis for trials started before the WHO TRDS extension. Also the “IPD Sharing Statement” was not included in the analysis for publications before the mandatory data sharing statement from July 1, 2018.24 In contrast, the “Completion Date” and “Basic Results” (comprises the current “Summary Results” item) items were required by the FDAAA 801 in ClinicalTrials.gov15 and were therefore analyzed. Additionally, the “All-cause Mortality” item in the Adverse Events (AEs) section was required by the Final Rule of the FDAAA, so we did not analyze this item for trials with a primary completion date before its implementation in 2017. We studied the reporting of deaths of such trials from other elements of the outcome data, primarily from Serious AEs (SAEs).
We determined the completeness and informativeness, i.e., missing information or uninformative terminology (unspecified or unclear information for the relevant registry item such as a code instead of a generic name of a drug), respectively, at first and last registration and in publications of WHO TRDS items.8,25 We used previous methods25 to evaluate the history of changes in WHO TRDS items from first to last registration and changes between last registration and corresponding publications. Changes were defined as qualitative (difference in the meaning of the provided information) or quantitative (difference in a numerical entry).25 We modified criteria developed by Chan et al.8 to describe discrepancies between registered and published outcomes as: a new primary outcome introduced in the article, an omitted registered primary or secondary outcome in the article, a registered primary or secondary outcome switched in the article or vice versa, a new secondary outcome introduced in the article, a registered primary or secondary outcome changed in the article, a new outcome methodology introduced or the registered one changed in the article, the timing of assessment changed in the article, and a new primary or secondary outcome introduced in the article as a combination of registered outcomes.
The completeness of results reporting from first to last registration and from last registration to the publication were determined by the presence of Participant Flow elements, Baseline Characteristics elements, Outcome Measures elements, and AEs.
Additionally, we analyzed these discrepancies in data reporting between trials with prospective and retrospective trial registration in ClinicalTrials.gov as well as between industry vs. non-industry sponsored trials. Trials with prospective registration started during or after first registration, and trials with retrospective registration started before it. We also analyzed the time differences between several points in the registration and publication.
Two investigators (IP and SP) independently extracted data in parallel from the entire cohort of trials for completeness, informativeness and changes to avoid potential data collector bias from possible subjective interpretation. Inter-rater reliability was high for changes in the WHO TRDS items (kappa range 0.83 to 1.00). We resolved through consensus discussion the differences in our interpretation of the secondary outcome changes, which had the lowest kappa in any single category (0.83, 95% confidence interval (CI) 0.73 to 0.94). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies.26 There was no need for approval from an institutional review board (Ethics Committee of the University of Split School of Medicine), as this study is a cross-sectional and historical cohort database study. We neither collected patient data nor performed experimental procedures.
Data extracted from ClinicalTrials.gov were coded and then entered into a Microsoft Excel spreadsheet. Dichotomous variables were registration period (retrospective or prospective), sponsorship (industry or non-industry) and completeness of registration (trial with or without missing registration items). The number of major changes and trial duration in months were treated as nonparametric, and were categorized based on a median split, where values were dichotomized as 0, when less than or equal to their respective median, and as 1 when greater than their respective medians. Differences in the trials, completeness, and major changes to the WHO TRDS items were compared using the Chi-square test. Frequencies, medians with the 95% CI were presented. Frequencies were compared using Chi-squared tests. We used IBM SPSS Statistics 21 (SPSS, Inc., an IBM company, Chicago, IL, USA, RRID:SCR_002865) for the analyses. Differences were considered significant at a P<.05.
A total of 76 trials were analyzed and 42 (55.3%) were published, two of which were in Japanese and one was only available as a Supplement. Thus, 39 (51.3%) publications were available for analysis. All were published in journals that follow ICMJE recommendations and only one (2.6%) was an ICMJE member.24 Further, there were 16 (41.0%) out of the 39 published trials where the corresponding publication was published before the results were posted in ClinicalTrials.gov[1]. Most trials in ClinicalTrials.gov were phase 3 (n=41 [53.9%]), quadruple-blind (n=33 [43.4%]), parallel group (n=64 [84.2%]) and placebo controlled (n=57 [75.0%]). In contrast, most published trials were double-blind (n=32 [82.1%]) (Table 1).
| First registration in ClinicalTrials.gov, n (%) | Last registration in ClinicalTrials.gov, n (%) | Publications, n (%) | ||
|---|---|---|---|---|
| Total=76 (100) | Total=76 (100) | Total=39 (100) | ||
| Phase | 1/2 | 2 (2.6) | 2 (2.6) | 0 (0.0) |
| 2 | 14 (18.4) | 14 (18.4) | 4 (10.3) | |
| 3 | 41 (53.9) | 41 (53.9) | 7 (17.9) | |
| 4 | 18 (23.7) | 19 (25.0) | 3 (7.7) | |
| Not provided | 1 (1.3) | 0 (0.0) | 25 (64.1) | |
| Masking | Open label | 6 (7.9) | 6 (7.9) | 3 (7.7) |
| Double blinded | 24 (31.6) | 24 (31.6) | 34 (87.2) | |
| Triple blinded | 12 (15.8) | 13 (17.1) | 1 (2.6) | |
| Quadruple blinded | 33 (43.4) | 33 (43.4) | 0 (0.0) | |
| Masking roles unspecified or missing | 1 (1.3) | 0 (0.0) | 1 (2.6) | |
| Control | Placebo | 56 (73.7) | 57 (75.0) | 27 (69.2) |
| Active | 8 (10.5) | 7 (9.2) | 3 (7.7) | |
| Both | 12 (15.8) | 12 (15.8) | 9 (23.1) | |
| Interventional model | Parallel | 62 (81.6) | 64 (84.2) | 24 (61.5) |
| Crossover | 10 (13.2) | 10 (13.2) | 7 (17.9) | |
| Single Group | 3 (3.9) | 2 (2.6) | 0 (0.0) | |
| Unspecified | 1 (1.3) | 0 (0.0) | 8 (20.5) | |
All 76 trials started before the implementation of the Final Rule and there were 22 (28.9%) trials with retrospective registration in ClinicalTrials.gov. Table 2 shows the median elapsed time between several points in the registration and publication of the trials. Most trials (n=64 [84.2%]) as well as most publications (n=36 [92.3%]) were industry-sponsored. We found that industry-sponsored compared to non-industry funded trials had a statistically significant shorter duration (χ2=6.848, P=.009).
| Median (95% CI) | |
|---|---|
| First registration in the ClinicalTrials.gov - Trial start date | -0.12 (95% CI -1.92 to 0.38) |
| Trial start date - Trial completion date (Trial duration) | 7 (95% CI 8.44 to 13.43) |
| Trial completion date - Date of posting results in the ClinicalTrials.gov | 18.1 (95% CI 20.31 to 29.09) |
| Trial completion date - Publication date | 22.9 (95% CI 23.28 to 32.12) |
| Date of posting results in the ClinicalTrials.gov - Publication date | 3.9 (95% CI -5.68 to 10.27) |
Table 3 shows which WHO TRDS items are most often missing at first and last registration and in publications. At least 1 WHO TRDS item was missing in 35 (46.1%) trials at first registration, in 15 (19.7%) at last registration and in 22 (56.4%) publications. There were statistically significantly more incomplete first registrations in trials with prospective registration than in those with retrospective (n=32 [91.4%] vs. n=3 [8.6%]); χ2=13.096, P≤.001.
| First registration in ClinicalTrials.gov, n (%) | Last registration in ClinicalTrials.gov, n (%) | Publications, n (%) | ||
|---|---|---|---|---|
| Trials missing WHO TRDS item, n (%) | 35 (46.1) | 15 (19.7) | 22 (56.4) | |
| WHO TRDS item | Scientific Title | 1 (1.3) | 0 (0.0) | 0 (0.0) |
| Countries of Recruitment | 26 (34.2) | 2 (2.6) | 5 (12.8) | |
| Key Inclusion and Exclusion Criteria | 0 (0.0) | 0 (0.0) | 1 (2.6) | |
| Study Type | 0 (0.0) | 0 (0.0) | 1 (2.6) | |
| Date of First Enrollment | 0 (0.0) | 0 (0.0) | 18 (46.2) | |
| Sample Size | 0 (0.0) | 0 (0.0) | 1 (2.6) | |
| Key Secondary Outcomes | 12 (15.8) | 13 (17.1) | 3 (7.7) | |
| Completion Date | 0 (0.0) | 0 (0.0) | 18 (46.2) | |
| IPD Sharing Statementa | / | / | 5 (83.3)b |
All registered trials had major changes in WHO TRDS items in the period between first and last registration as well as all publications (Tables 4-5 and Supporting Tables 1-3 in Extended data). The median major changes per trial between first and last registration was 31 (95% CI 35.73 to 65.29), and per publication it was 28 (95% CI 24.6 to 32.2) (Table 4). There was no difference in the number of major changes to WHO TRDS items per trial between first and last registration. as well as between last registration and publication according to prospective or retrospective trial registration; χ2=3.003, P=.083 and χ2=2.820, P=.093, respectively.
Predominantly, methodological details were changed in both primary and secondary outcomes between first and last registration as well as in publications (Table 5). Furthermore, primary outcome changes between first and last registration and in publications included the addition of new outcomes and changes to existing ones. According to prospective or retrospective trial registration, we found no difference in the number of major changes to primary and secondary outcomes between first and last registration as well as between last registration and publication; χ2=0.616, P=.433; χ2=2.758, P=.097; χ2=2.201, P=.138 and χ2=2.820, P=.093, respectively.
We found uninformative reporting of WHO TRDS items in ClinicalTrials.gov and publications (Table 6). The median uninformative WHO TRDS items per trial at first registration was 3 (95% CI 3.1 to 3.8), 1 (95% CI 1.3 to 1.9) at last registration, while in publications it was 0 (95% CI 0.08 to 0.43). At first registration, most trials had uninformative “Primary and Key Secondary Outcomes” and “Intervention(s)” items. The outcomes were uninformative mainly due to their unclear methodology that was clarified by last registration, and the interventions mainly due to a missing time frame (Table 6). Table 6 also shows that registered and published interventions from RCTs on AR had uninformative descriptions. 28 (71.8%) publications had a less informative “Study Type” than in ClinicalTrials.gov, mostly due to omitted study phase (Supporting Table 3 in Extended data).
| Uninformative WHO TRDS item | Trials with uninformative item, n (%) | Uninformativeness (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| Code instead of drug name | Unclear methodology | Unexplained abbreviation | Non-lay terminology | Unclear or too short | Time frame missing | Dosage missing | ||
| First registration in ClinicalTrials.gov | Total 73 (96.1) | |||||||
| Public Title | 29 (38.2) | 12 | 5 | 6 | 6 | |||
| Scientific title | 18 (23.7) | 12 | 6 | |||||
| Intervention(s) | 59 (77.6)a | 11 | 31 | 6 | 39 | 9 | ||
| Key Inclusion and Exclusion Criteria | 31 (40.8) | 2 | 29 | |||||
| Primary Outcome(s) | 66 (86.8)a | 66 | 1 | |||||
| Key Secondary Outcomes | 55 (72.4) | 55 | ||||||
| Last registration in ClinicalTrials.gov | Total 60 (78.9) | |||||||
| Public Title | 25 (32.9) | 11 | 3 | 6 | 5 | |||
| Scientific title | 16 (21.1) | 11 | 5 | |||||
| Intervention(s) | 47 (61.8)a | 7 | 24 | 5 | 1 | 30 | 5 | |
| Key Inclusion and Exclusion Criteria | 26 (34.2) | 2 | 24 | |||||
| Primary Outcome(s) | 3 (3.9) | 3 | ||||||
| Key Secondary Outcomes | 5 (6.6) | 5 | ||||||
| Corresponding publications | Total 4 (10.3) | |||||||
| Scientific title | 1 (2.6) | 1 | ||||||
| Intervention(s) | 1 (2.6) | 1 | ||||||
| Primary Outcome(s) | 1 (2.6) | 1 | ||||||
| Key Secondary Outcomes | 2 (5.1) | 1 | 1 | |||||
Regarding participant characteristics, all trials in ClinicalTrials.gov reported Participant overall flow and Baseline population descriptions while only 1 (2.6%) out of 39 publications did not. All 76 trials reported SAEs and Other AEs (OAEs) in ClinicalTrials.gov. In contrast, 30 (76.9%) publications reported SAEs and 38 (97.4%) OAEs. Due to primary completion dates after 2017, the reporting of the “All-cause Mortality” item was analyzed for only nine (11.8%) trials, all of which have a reported item in ClinicalTrials.gov. Of these, four were published and two of them reported deaths. There were only two (2.6%) trials that reported deaths, both as an SAE. Of these, both were published, and only one publication reported it. Overall, deaths were reported in 20 (51.3%) publications.
Our novel cross-sectional study on the discrepancies in the reporting of data from RCTs on AR showed that most of the analyzed trials were phase 3 and 4. These studies, especially if published in high-impact journals, have the greatest impact on clinical care and formulate clinical practice guidelines.20 For most trials in ClinicalTrials.gov (n=45 [59.2%]) masking/blinding was listed as quadruple and triple. In contrast, most publications were double-blind (n=34 [87.2%]), and only one (2.6%) publication was listed as triple-blind, while none were listed as quadruple. This discrepancy in the reporting of masking/blinding between registered protocol data and corresponding publications and insufficient descriptions of the blinding process in publications is contrary to the updated Consolidated Standards of Reporting Trials (CONSORT) guidelines, which require transparent reporting of this process.27,28
Almost half of the registered trials had incomplete first registration. Furthermore, more than a quarter of trials had retrospective registration in ClinicalTrials.gov, and those trials also had a more complete first registration. Perhaps researchers or sponsors perceive a greater incentive to fully register when the trial is progressing significantly or about to be published rather than to prospectively register the trial.29
More than half of the publications also had missing registration items that differed significantly from those in ClinicalTrials.gov. In addition to poor reporting of deaths, also almost a quarter of the publications were missing explicit reporting of SAEs. The analysis of discrepancies in the reporting of deaths between ClinicalTrials.gov and publications is deficient due to the fact that the item "All-cause Mortality" was required by the implementation of the Final Rule and therefore included only nine (11.8%) trials. In contrast, we could not associate the implementation of the Final Rule with the publication of trial results prior to their posting in ClinicalTrials.gov due to the fact that only two (5.1%) trials were published before April 2017 from which responsible parties have been required to be compliant, and the posting of the corresponding results in ClinicalTrials.gov was after that date.23
All trials in ClinicalTrials.gov as well as published trials had major changes to registration items mainly due to the aggregate nature of the changes (e.g., deletions, additions, or changes to the outcome's methodology). A concerning fact is that changes in outcomes, study design and interventions were among the most common. We expected changes to the WHO TRDS items for prospectively registered trials,25,29 but if without approval from an ethics committee, changes to primary and secondary outcomes in more than 80% of the trials between first and last registration as well as changes to primary outcomes in 26% and secondary in 82% of the corresponding publications are unjustifiable.
The presence of uninformative WHO TRDS items clearly declined between first registration and publication, especially between last registration and publication. Of particular concern is the significant prevalence of uninformative outcomes as well as interventions at first registration.
Accordingly, it can be concluded that journals following ICMJE recommendations do not consider registration data inadequate if any WHO TRDS item is missing or uninformative.12 To address this issue, Talebi et al. proposed the introduction of a registration checklist for researchers that would provide an explanation in the publication of any discrepancy related to registered information and request a link to the corresponding ClinicalTrials.gov record to help journal editors find discrepancies between registered data and data in submitted manuscripts.20
Concerning the primary sponsor, our findings that most of the trials (n=64 [84.2%]) were industry-sponsored and significantly shorter are consistent with the results of a cross-sectional study conducted on a sample of 245,999 registered interventional studies in ClinicalTrials.gov.30 Although we found a predominance of industry-sponsored trial publications, less than half (n=36 [47.4%]) of them were published, while slightly higher percentages (55-68%) of industry-sponsored publications were reported in the literature.31,32 Notably in ClinicalTrials.gov, the primary sponsor data item is inseparable from the funding source, a WHO data item that is not included in the current Administrative Information section of a trial record. Thus, administrators at ClinicalTrials.gov may need to introduce a separate “Source(s) of Monetary or Material Support” field to improve comparison and reduce the risk of misclassification of funders.30
We analyzed trials only registered in ClinicalTrials.gov, which is a limitation to the study. There are currently 17 other primary registries in the WHO registry network that meet ICMJE requirements and therefore our analyzed data may not have external validity to trials registered outside of ClinicalTrials.gov.33 However, we used ClinicalTrials.gov, which is the largest clinical trial registry.5,10 Another limitation could be the oversight of some of the existing publications despite different search methods. Furthermore, data interpretation may be subjective, particularly regarding major changes in the WHO TRDS items and results due to data collector characteristics and bias. Therefore, a second reviewer (SP) independently extracted data and we performed inter-rater agreement, and through discussion and consensus, we determined clear rules for data entry to minimize subjective data interpretation. Finally, our samples regarding RCTs and publications were small so the changes or discrepancies recorded should be viewed with caution.
Despite the WHO and ICMJE registration requirements and US legal provisions, the completeness and consistency of WHO TRDS items of RCTs on AR registered in ClinicalTrials.gov as well as in the corresponding publications is mostly poor. The informativeness of the analyzed items is higher in publications than in ClinicalTrials.gov. Whichever of the WHO TRDS items are applicable to trials, trialists should ensure that the registered data is accurate and subsequent checks could ensure this before the publication of AR trial data. Checking the accuracy of trial data through multiple sources and resolving any discrepancies before final approval for publication would help prevent publication bias and improve the transparency of data reporting.
Open Science Framework: Cross-sectional study on the reporting of allergic rhinitis trials registered in ClinicalTrials.gov and in corresponding publications.
https://DOI: doi.org/10.17605/OSF.IO/YMHC2.
This project contains the following underlying data:
- Data file 1: Paladin_Database_OSF
- Data file 2: ClinicalTrials.gov database coding key
- Data file 3: Extended data/Supporting Tables
Data associated with the article are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
All authors meet the ICMJE authorship criteria.
Ivan Paladin: data acquisition and interpretation, statistical analysis, writing and revising of the manuscript, approval of the final version.
Shelly Melissa Pranić: design and supervision of the study, data acquisition and interpretation, critical review, revision and approval of the final version of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Venous leg ulcers; trial methodology.
At the request of the author(s), this article is no longer under peer review.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 13 Jun 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)