Keywords
sofosbuvir, daclatasvir, ledipasvir, chronic hepatitis C, lipid profile, glycemic status, sustained virological response, end of treatment
This article is included in the Pathogens gateway.
sofosbuvir, daclatasvir, ledipasvir, chronic hepatitis C, lipid profile, glycemic status, sustained virological response, end of treatment
AEs: adverse effects
ALT: alanine aminotransferase
APO B: apolipoprotein B
AST: aspartate aminotransferase
ASV: asunaprevir
BMI: body mass index
CBC: complete blood picture
CHC: chronic hepatitis C
cm: centimeters
CT: computerized tomography
DAAS: directing acting antivirals
DAC: daclatasvir
dL: deciliters
EOT: end of treatment
FBS: fasting blood sugar
FIB-4: fibrosis-4 index
HbA1c: gylcated hemoglobin
HBV: hepatitis B virus
HCC: hepatocellular carcinoma
HCV: hepatitis C virus
HDL: high-density lipoproteins
HOMA-IR: homeostatic model assessment of insulin resistance.
IFN: interferon
INR: international normalized ratio
IR: insulin resistance
IRS-1: insulin receptor substrate-1
IU: international units
kg: kilograms
LDL: low-density lipoproteins
LED: ledipasvir
mg: milligrams
mm3: cubic millimeters
MRI: magnetic resonance imaging
NCCVH: National Committee for the Control of Viral Hepatitis
PCR: polymerase chain reaction
PEG-IFN: pegylated interferon
RBV: ribavirin
RNA: ribonucleic acid
SD: standard deviation
SIM: simeprevir
SOF: sofosbuvir
SVR12: sustained virological response week 12
T2DM: type-2 diabetes mellitus
TC: total cholesterol
TG: triglycerides
TLC: total leucocytic count
VLDL: very low-density lipoproteins
Hepatitis C virus (HCV), a blood borne disease, is a primary causative agent of chronic liver diseases such as fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) that lead to related high mortality and morbidity.1 Several lipid and lipoprotein metabolic pathway disorders such as hypocholesterolemia, hepatic steatosis, hypobetalipoproteinemia that cause fatty liver and insulin resistance (IR), are associated with chronic HCV infection.2 IR occurs in the early phases of HCV infection and can manifest later on as type-2 diabetes mellitus (T2DM), which can accelerate the progression of liver fibrosis into cirrhosis and later on into HCC.3
Globally, about 170 million patients with HCV chronic liver disease have been reported.1 Egypt has the greatest prevalence of HCV globally with approximations more than 10% amongst the overall inhabitants with genotype-4 being the most dominant type in 91% of the cases.4 The HCV large infection pool in Egypt was believed to be caused by the national parenteral anti-schistosomiasis campaign in the late 1860s–1980s. Moreover, 24.3% of HCV transmission was due to a history of previous blood transfusions, 20.6% due to needle reuse, medical procedures, and household transmissions.5
The treatment of chronic HCV disease has shifted from interferon (IFN)-based into IFN-free regimens consisting of directly acting antiviral agents. Second generation direct acting antivirals show sustained virological response (SVR) rates ≥ 90% with reduced side effects and increased tolerability.6–10 Treatment with direct acting antiviral (DAA) leads to improvement in the rates of SVR and decreases the predictive aptitude of baseline metabolic characteristics for SVR with IFN-based treatment, even though baseline low-density lipoprotein (LDL) is still a predictor in some direct acting antiviral (DAA) studies.7
Hepatitis C virus replication and maturation have shown a robust interaction between the virus and host intracellular lipids, indicating that host lipid metabolism and cholesterol homeostasis have a crucial role in HCV circulation and HCV replication cycle stages including entry, replication, assembly, and secretion.11,12
Pathophysiological mechanisms for IR by HCV include direct and indirect pathways. HCV proteins have a direct influence on inhibiting the insulin-signaling pathway stimulating central IR through increasing the insulin receptor substrate-1 (IRS-1) degradation by preventing IRS1 from binding to insulin receptors and hence impairing glucose metabolism.7,12 Peripheral IR occurs as a result of indirect pathways due to overproduction of inflammatory cytokines and high lipolysis by HCV.7,13
Chronic hepatitis C (CHC) is associated with decreased serum levels of LDL, total cholesterol (TC), and apolipoprotein B (APO B) since HCV utilizes host circulating lipids for their replication causing hypolipidemia. In addition, it is associated with increased IR and T2DM rates.14,15 In the era of pegylated interferon (PEG-IFN) plus ribavirin (RBV), several studies reported an increase in LDL, TC, and triglycerides (TG) after achieving SVR suggesting that HCV has a significant role in baseline hypolipidemia.16–20
Lipid and glucose metabolic changes data are controversial regarding direct acting antivirals (DAAS).15 Some studies reported a significant increase in TC and LDL irrespective of DAAS treatment regimen type and HCV genotype suggesting an interrelationship between HCV and host lipid metabolism which reverses to baseline levels after failure of treatment.9,21
However, this was opposed by other studies22,23 where they found significant differences in lipid changes between different DAA regimens. This finding indicates that successful viral elimination is not the only factor that affects lipid levels during treatment with DAAS, but DAAS themselves may have pharmacological effects on host lipid metabolism. Hence, increases in lipids are strongly dependant on antiviral DAAS type.22,23
Successful viral eradication by DAAS showed improvements in glycemic control with worsening of lipid parameters due to alterations of glucose and lipid metabolic pathways by HCV. Increase in lipid profile might be due to the return of lipids to normal pre-virological levels since HCV is known to cause hypolipidemia. However, these alterations might have a potential cardiovascular risk that might require monitoring during DAA treatment.15
Several studies investigated metabolic changes in lipid metabolism and glycemic control for other genotypes, but it has not been studied extensively in genotype-4 patients. This observational study aimed to assess the outcome of using sofosbuvir/daclatasvir and sofosbuvir/ledipasvir as treatment regimens for chronic hepatitis C genotype-4 infected non-diabetic non-cirrhotic Egyptian patients and their impact on lipid profile and glycemic control.
This prospective observational cohort study was conducted on 140 easy-to-treat treatment-naïve genotype-4 chronic HCV infected patients diagnosed by a positive test of anti-HCV antibodies and HCV RNA. Patients were recruited from the outpatient clinics of Al-Demerdash Ain Shams University Hospital hepatitis viral unit (one of the centers of the National Committee for the Control of Viral Hepatitis) between the periods of October 2019 to April 2020.
The study protocol was approved by the ethical committee of Ain Shams University, Faculty of Pharmacy, Cairo, Egypt (ID number 164). This study was conducted according to the Declaration of Helsinki ethical guidelines for medical research involving humans and good clinical practice standards.24 A written informed consent was obtained from each patient without any obligations and the patients were able to withdraw at any time if they wanted to.25
Patients were diagnosed with chronic HCV infection by showing continuous anti-HCV antibody and HCV-RNA positivity by quantitative polymerase chain reaction (PCR) for six months or more. Patients included in the study were those who fulfilled the criteria of the National Committee for the Control of Viral Hepatitis (NCCVH) in Egypt for treatment. It included male or female easy-to-treat, treatment-naïve patients with an age range between 18 and 75 years old. Treatment-naïve was defined as patients who had not undergone any previous HCV treatment or relapsed from previous DAAS or pegylated-interferon plus ribavirin treatment. Easy-to-treat was defined as patients who were treatment-naïve, non-cirrhotic chronic HCV with compensated biochemical liver parameter26 categorized by certain labs according to the National Committee for the Control of Viral Hepatitis such as serum biliribun ≤ 1.2 mg/dL, serum albumin ≥ 3.5g/dL, international normalized ratio (INR) ≤ 1.2, and platelet count ≥ 150,000/mm3. Patients’ liver condition was assessed by fibrosis-4 index (FIB-4) where FIB-4 index score of > 3.25 predicts significant liver fibrosis (F3–F4). Pelvic abdominal ultrasonography was routinely performed on all patients to assess hepatic status and further advanced imaging modalities, such as computerized tomography (CT) or magnetic resonance imaging (MRI), were performed when needed.
Patients not eligible for HCV treatment according to the National Committee for the Control of Viral Hepatitis with any of the following criteria were excluded: Child’s C cirrhotic patients (score ≥ 9); platelet count < 50,000/mm3; patients with active hepatocellular carcinoma (HCC) except for those who were cured for more than six months with no active evidence of HCC by dynamic imaging (CT or MRI); patients with active extra-hepatic malignancies except for those who were malignancy free for a time period of two years or patients who were cured from lymphomas and chronic lymphocytic leukemia and could start treatment immediately after remission based on the oncology report; pregnancy or patients who were unable to use contraceptives; uncontrolled diabetes mellitus with glycated hemoglobin levels (HbA1c > 9%).
Exclusion criteria in this study also included patients with factors that may affect lipid profiles or glycemic control. Factors that were excluded by examination and medical history profile included lipid lowering medications (statins); endocrine diseases such as hyperthyroidism or hypothyroidism; diabetes mellitus; Cushing’s disease; patients receiving hypertensive medication or corticosteroids that may induce insulin resistance; other comorbidities such as renal disease. Chronic HCV patients suffering from comorbid liver diseases such hepatitis B virus (HBV) or human immunodeficiency (HIV) coinfections, hepatocellular carcinoma or autoimmune hepatitis were also excluded from our study.25 Diabetic patients were defined by the American Diabetes Association guidelines as those with fasting glucose of ≥ 126 mg/dL or HbA1C of ≥ 6.5%.27
Three hundred patients were assessed and only one hundred and forty patients fulfilled the inclusion criteria and were included in the study. Patients included received either SOF/DAC regimen 400 mg sofosbuvir (Sovaldi® by Gilead Sciences INC., Foster City, CA) plus daclatasvir 60 mg (Daclavirocyrl by MARCYRL pharmaceutical industries, Egypt) daily for 12 weeks28 or SOF/LED regimen 400 mg sofosbuvir plus ledipasvir 90 mg (a fixed-dose combination tablet named Harvoni® by Gilead Sciences, Foster City, CA) daily for 12 weeks.28 Treatment regimens were determined and prescribed by physicians in charge at Al-Demerdash Ain Shams University hospital. Both treatment regimens are readily available and prescribed at Al-Demerdash Ain Shams University Hospital. No intervention was done regarding HCV treatment plan decided for patients by hospital physicians. Figure 1 shows the study flow chart methodology.
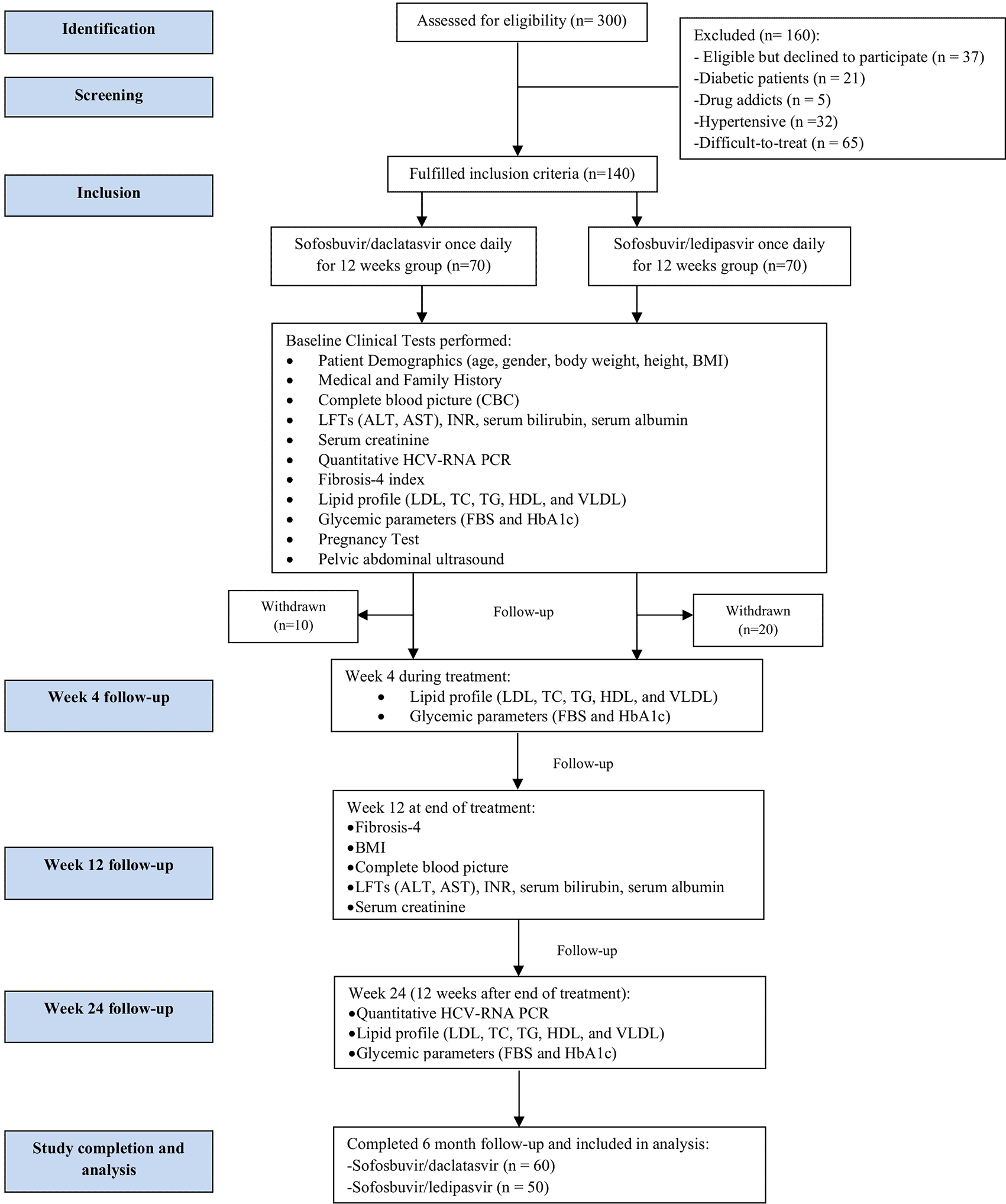
BMI: body mass index; CBC: complete blood picture; INR: international normalized ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase; HCV: hepatitis C virus, RNA: ribonucleic acid, PCR: polymerase chain reaction, LDL: low-density lipoproteins, TC: total cholesterol, TG: triglycerides, HDL: high density lipoproteins, VLDL: very low-density lipoproteins, FBS: fasting blood sugar, HbA1c: glycated hemoglobin, LFTs: liver function tests.
Before starting treatment, all patients underwent a full medical assessment, medication history, and family history. Patient demographics such as age, sex, body weight (kg), height (cm), and calculated BMI (body mass index) were collected. Complete laboratory investigations including complete blood count (CBC); liver function tests such as alanine aminotransferase (ALT), aspartate aminotransferase (AST); international normalized ratio (INR); serum bilirubin; serum creatinine; serum albumin; baseline quantitative HCV-RNA PCR; calculated fibrosis-4 (FIB-4) index; complete lipid profile including fasting low-density lipoprotein (LDL), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL); glycated hemoglobin (HbA1c); fasting blood sugar (FBS).25
Patients were followed-up during treatment for 12 weeks and after the end of treatment (EOT) for 12 weeks (total duration of 24 weeks follow-up). Total lipid profile, fasting blood sugar, and HbA1c were measured at four weeks (during treatment), and 12 weeks post-treatment (at 24 weeks). CBC, liver function tests, serum albumin, serum bilirubin, serum creatinine, and INR were measured at week 12. BMI and FIB-4 were calculated at EOT at week 12 of treatment.25
Sustained virological response 12 (SVR 12) is defined as undetectable HCV ribonucleic acid (RNA) by polymerase chain reaction (PCR) after 12 weeks of completion of HCV therapy.29 HCV-RNA was obtained at baseline (before the start of therapy) and at week 24 after completion of therapy by 12 weeks. HCV-RNA levels were quantified using Roche Diagnostics TaqMan PCR V.2.0, Mannheim, Germany (lower detection limit: 15 IU/ml).30 The study main end point was considered undetected HCV-RNA after the EOT by 12 weeks known as SVR 12.25
Patients were asked to report any adverse effects (AEs) due to treatment during the entire study period and follow-up. Safety end points that would stop continuation of a patient’s treatment included severe AEs, laboratory abnormalities, discontinuation of treatment due to AEs intolerance, and death. Safety was ensured during the study period by assessing monthly laboratory hematological and biochemical parameters along with AEs reporting.
Enrolment was dependent on the clinical need for medication rather than on statistical factors. Sample size was estimated using nQuery statistical package, version 7.0 (Los Angeles, CA); an open-access alternative is G*Power software (RRID: SCR_013726). Statistical analysis was done using IBM SPSS® Statistics version 22 (IBM® Corp., Armonk, NY, USA) (RRID:SCR_019096); an open-access alternative is JASP version 0.16.1 (RRID: SCR_015823). Numerical data were tested for normality using Kolmogorov-Smirnov test and Shapiro-Wilk test. For quantitative data, comparison between two groups was done using the Student t-test for normally distributed data; while for non-normally distributed data, the Mann-Whitney test (non-parametric t-test) was used. Comparison between two groups was done using the Kruskal-Wallis test (non-parametric ANOVA) then the post-Hoc test was used for pair-wise comparison based on the Kruskal-Wallis distribution. Wilcoxon-signed ranks test (non-parametric paired t-test) was used to compare two consecutive measures of numerical variables. Friedman’s test (non-parametric ANOVA with repeated measures) was used to compare more than two consecutive measures of numerical variables. All tests were two-tailed. A p-value < 0.05 was considered significant.
In previous studies, Hashimoto et al., 2016 and Jain et al., 201923,31 showed a difference in ΔLDL (percentage change LDL) between SOF/LED and SOF/DAC of 13 mg/dL with a pooled standard deviation of 21. Based on these findings, a minimal sample size of 42 subjects in each group was required at an alpha level of 0.05 and power of 80%. To compensate for loss to follow-up, the sample size was increased by 70% to 70 subjects in each group with a total sample size of 140 subjects.
Out of 140 patients who fulfilled the inclusion criteria and started the study, only 110 patients completed the whole study period and were included in the final analysis. The first group included 60 patients who received the SOF/DAC regimen and the second group included only 50 patients who received the SOF/LED regimen. The reason for the patients’ withdrawal or exclusion after starting participation was noncompliance to study protocol due to personal reasons (as shown in Figure 1). Patients in both groups achieved a 100% sustained virological response (SVR).25
In the SOF/DAC group, the mean age was 46.7 years. The 60 patients who received SOF/DAC were 23 (38.3%) males and 37 (61.7%) females, and their mean BMI was 30.1. While the mean age of the 50 patients who received SOF/LED treatment was 54.8. The males represented 24 (48.0%) patients and 26 (52.0%) were females, and their mean BMI was 25.1. The baseline demographic and laboratory parameters are presented in Table 1. A significant difference was found between the patients in the two groups regarding many baseline characteristics and laboratory parameters. Therefore, further comparisons were done using percentage change between both groups.
| SOF/DAC group n=60 | SOF/LED group n=50 | P-value | |
|---|---|---|---|
| Age (years) [mean ± SD]a | 46.7 ± 13.4 | 54.8 ± 7.6 | < 0.001* |
| Sex | |||
| Male [n (%)]b | 23 (38.3%) | 24 (48.0%) | 0.307 |
| Female [n (%)]b | 37 (61.7%) | 26 (52.0%) | |
| Body mass index (kg/m2) [mean ± SD]a | 30.1 ± 6.7 | 25.1 ± 4 | < 0.001* |
| Hemoglobin (g/dL) [mean ± SD]a | 13.9 ± 1.4 | 13.6 ± 1.7 | 0.308 |
| TLC (×103/mm3) [mean±SD]a | 7.8 ± 2.1 | 6.9 ± 2.4 | 0.020* |
| Platelet count (×103/mm3) [mean ± SD]a | 251.9 ± 70.2 | 207.8 ± 71.1 | 0.001* |
| ALT (IU/ml) [median (range)]c | 33.5 (15.0–346.0) | 43.5 (7.0–150.0) | 0.507 |
| AST (IU/ml) [median (range)]c | 32.5 (10.0–254.0) | 42.0 (12.0–234.0) | 0.049* |
| Bilirubin (mg/dL) [mean ± SD]a | 0.65 ± 0.23 | 0.77 ± 0.29 | 0.023* |
| Albumin (mg/dL) [mean ± SD]a | 4.3 ± 0.5 | 4.1 ± 0.4 | 0.016* |
| INR [mean ± SD]a | 1.08 ± 0.07 | 1.08 ± 0.11 | 0.943 |
| Fibrosis-4 score [mean ± SD]a | 1.2.0 ± 0.62 | 2.14 ± 1.21 | < 0.001* |
| Creatinine (mg/dL) [mean ± SD]a | 0.83 ± 0.18 | 0.88 ± 0.22 | 0.199 |
| Total cholesterol (mg/dL) [mean ± SD]a | 143.7 ± 21.8 | 157.6 ± 24.8 | 0.004* |
| Triglycerides (mg/dL) [mean ± SD]a | 107.3 ± 24.4 | 124.7 ± 37.2 | 0.005* |
| HDL (mg/dL) [mean ± SD]a | 46.2 ± 3.5 | 44 ± 12.4 | 0.344 |
| LDL (mg/dL) [mean ± SD]a | 76.4 ± 21.6 | 88.6 ± 20.6 | 0.005* |
| VLDL (mg/dL) [mean ± SD]a | 21.5 ± 4.9 | 24.9 ± 7.4 | 0.005* |
| Fasting blood sugar (mg/dL) [mean ± SD]a | 106.4 ± 13.2 | 97.5 ± 13.7 | 0.128 |
| HbA1c (%) [mean ± SD]a | 5.4 ± 0.68 | 5.9 ± 0.21 | < 0.001* |
Twelve weeks after treatment, the two groups showed a significant decrease in hemoglobin concentration with p-value < 0.001 and 0.002 in SOF/DAC and SOF/LED respectively. Meanwhile, no significant change of TLC or platelet count after treatment in both groups was detected. The body mass index did not change significantly at end of treatment in both groups with p-value 0.871.
On the other hand, ALT, AST, and FIB-4 score decreased significantly with baseline 43.5 (7–150) versus end of treatment 18 (8–70), 42 (12–234) versus 21 (9–54), and 1.91 (0.45–5.61) versus 1.3 (0.42–6.58) respectively at the end of treatment in the SOF/LED group. Moreover, this reduction was significantly higher in the SOF/LED group compared to the SOF/DAC group with p-value < 0.001 (as shown in Table 2).
| Parameter | SOF/DAC Group | SOF/LED Group | P-value | ||||
|---|---|---|---|---|---|---|---|
| Median | Min. | Max. | Median | Min. | Max. | ||
| Body mass index (kg/m2) | -0.3 | -31.8 | 29.4 | 0.3 | -4.1 | 48.4 | 0.871 |
| TLC (×103/mm3) | -4.7 | -60.0 | 48.4 | 1.6 | -59.4 | 110.6 | 0.440 |
| Hemoglobin (g/dL) | -5.9 | -44.9 | 22.2 | -3.3 | -25.3 | 13.0 | 0.088 |
| Platelet count (×103/mm3) | -2.7 | -60.7 | 63.7 | -0.1 | -48.9 | 84.3 | 0.245 |
| ALT (IU/ml) | 0.0 | -88.4 | 200.0 | -55.5 | -92.2 | 185.7 | < 0.001* |
| AST (IU/ml) | 0.0 | -84.3 | 205.9 | -49.4 | -90.7 | 77.3 | < 0.001* |
| Bilirubin (mg/dL) | 0.0 | -71.4 | 300.0 | -12.1 | -96.0 | 133.3 | 0.195 |
| Albumin (mg/dL) | -2.3 | -40.3 | 24.4 | 0.0 | -13.3 | 28.6 | 0.152 |
| Creatinine (mg/dL) | 8.1 | -42.9 | 55.6 | 0.0 | -60.8 | 225.0 | 0.516 |
| INR | -2.7 | -21.3 | 24.0 | 0.0 | -23.1 | 58.3 | 0.665 |
| Fibrosis-4 score | 5.0 | -73.5 | 263.9 | -23.4 | -85.6 | 141.2 | 0.001* |
The two groups experienced a similar pattern of change in total cholesterol levels throughout the treatment period. In the SOF/DAC and SOF/LED groups, cholesterol increased significantly after four weeks from baseline levels with (150.1 ± 31.9) versus (143.7 ± 21.8) and (175.1 ± 23.0) versus (157.6 ± 24.8) respectively with p-value < 0.001. In week 24, the TC decreased significantly in both groups compared to week four with (143.0 ± 28.8) and (169.9 ± 21.5) with p-value < 0.001 in SOF/DAC and SOF/LED groups respectively but remained higher than baseline levels. Moreover, the percentage change of TC levels was significantly different between the two groups (as shown in Table 3).
| Parameter | SOF/DAC Group | SOF/LED Group | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Median | Min. | Max. | Median | Min. | Max. | |||
| Total cholesterol (mg/dL) | Base to W4 | -2.4 | -36.6 | 76.0 | 12.2 | -4.9 | 33.9 | 0.007* |
| Base to W24 | -102.5 | -123.1 | -33.4 | -95.7 | -104.2 | -79.9 | 0.008* | |
| W4 to W24 | -102.5 | -136.5 | -62.2 | -96.1 | -104.0 | -81.0 | 0.007* | |
| Triglycerides (mg/dL) | Base to W4 | 7.1 | -31.3 | 76.0 | -1.4 | -38.8 | 27.3 | 0.074 |
| Base to W24 | -7.5 | -40.3 | 77.6 | 3.4 | -16.9 | 40.9 | 0.001* | |
| W4 to W24 | -9.2 | -34.9 | 26.6 | 4.6 | -26.5 | 70.4 | < 0.001* | |
| LDL (mg/dL) | Base to W4 | -7.7 | -56.7 | 198.9 | 19.6 | -9.2 | 51.7 | 0.005* |
| Base to W24 | -4.3 | -61.3 | 191.5 | 11.9 | -8.8 | 59.6 | 0.026* | |
| W4 to W24 | -4.2 | -37.5 | 149.0 | -7.9 | -15.9 | 35.7 | 0.059 | |
| HDL (mg/dL) | Base to W4 | -2.1 | -24.1 | 23.1 | 0.0 | -19.5 | 30.0 | 0.867 |
| Base to W24 | -4.3 | -29.3 | 11.6 | -5.8 | -25.6 | 32.1 | 0.919 | |
| W4 to W24 | -4.4 | -10.4 | 13.2 | -5.6 | -30.5 | 54.2 | 0.668 | |
| VLDL (mg/dL) | Base to W4 | 7.1 | -31.3 | 76.0 | -1.4 | -38.8 | 27.3 | 0.074 |
| Base to W24 | -7.5 | -40.3 | 77.6 | 3.4 | -16.9 | 40.9 | 0.001* | |
| W4 to W24 | -9.2 | -34.9 | 26.6 | 4.6 | -26.5 | 70.4 | < 0.001* | |
Regarding the change in TG levels in the SOF/DAC group, TG increased significantly after four weeks (113.4 ± 28.4) from the baseline levels (107.3 ± 24.4) with p-value < 0.028, then decreased significantly after 24 weeks (99.2 ± 18.5) with p-value < 0.001. In the SOF/LED group, TG did not change significantly after four weeks of treatment (123.7 ± 36.8) compared to baseline readings (124.7 ± 37.2) with p-value = 1.00, but it increased significantly after 24 weeks (130.7 ± 40.2) compared to the week-four reading with p-value 0.032. The percentage change of triglyceride levels was significantly different between the two groups (as shown in Table 3).
The change in LDL levels was also similar in both groups where in the SOF/DAC group, LDL increased after four weeks (81.6 ± 31.1) from the baseline levels (76.4 ± 21.6) but not significantly with p-value = 0.215. While in week 24, the LDL decreased significantly (79.3 ± 27.9) compared to week four with p-value = 0.01. In the SOF/LED group, LDL increased significantly after four weeks (106.7 ± 20.0) versus the baseline levels (88.6 ± 20.6) with p-value < 0.001 and then decreased significantly after 24 weeks (101.8 ± 17.4) with p < 0.015 but remained higher than the baseline levels. Also, the percentage change of LDL was significantly different between the two groups (as shown in Table 3).
In the SOF/DAC group, HDL levels did not change significantly after four weeks (45.8 ± 3.1) from the baseline levels (46.2 ± 3.5) with p-value = 1.00, but it decreased significantly after 24 weeks (43.9 ± 2.3) with p-value < 0.001. In the SOF/LED group, HDL levels did not change significantly after four weeks of treatment (43.6 ± 10.4) or after treatment at week 24 (41.9 ± 9.0) from the baseline levels (44.0 ± 12.4) with p-value = 0.127. Also, the percentage change of HDL levels was comparable between the two groups (as shown in Table 3).
Regarding the glycemic parameters, both HbA1c and FBS decreased throughout the study period in both groups (as shown in Figures 2 and 3). In the SOF/DAC group, HbA1c did not decrease significantly after four weeks (5.2 ± 0.6) from baseline levels (5.4 ± 0.68) with p-value = 0.166, but it decreased significantly at week 24 (5.0 ± 0.57) from baseline with p-value < 0.001. In the SOF/LED group, there was a significant decrease at week four and week 24 (5.3 ± 0.35) and (5.2 ± 0.33) respectively compared to baseline levels (5.9 ± 0.21) with p-value <0.001. Regarding FBS, the SOF/DAC group showed no significant decrease at week four (99.2 ± 12.2) with p-value = 0.32 from baseline (106.4 ± 13.2), but significantly decreased at week 24 (91.2 ± 12.5) with p-value < 0.001. In the SOF/LED group, FBS showed no significant decrease at week four (96.4 ± 12.5) with p-value = 1, but significantly decreased from baseline at week 24 (91.9 ± 11.3) with p-value = 0.049. There was a significant difference between the two groups in both percentage change of HbA1c and FBS. There was a significant percentage change of HbA1c between both groups at week four and week 12 with p-value < 0.001 and p-value = 0.032 respectively from baseline values with higher change in the SOF/LED group. Regarding FBS, there was a significant percentage change between both groups at week four and week 24 with p-value = 0.014 and p-value < 0.001 respectively from baseline values with higher percentage change in the SOF/DAC group.25

SOF/DAC: sofosbuvir/daclatasvir, SOF/LED: sofosbuvir/ledipasvir, week 4: during treatment, week 24: 12 weeks post end of treatment. Data is expressed as mean ± SD. Friedman’s test is used for statistical significance non-parametric data comparisons with repeated measures. P-value < 0.05 is statistically significant.
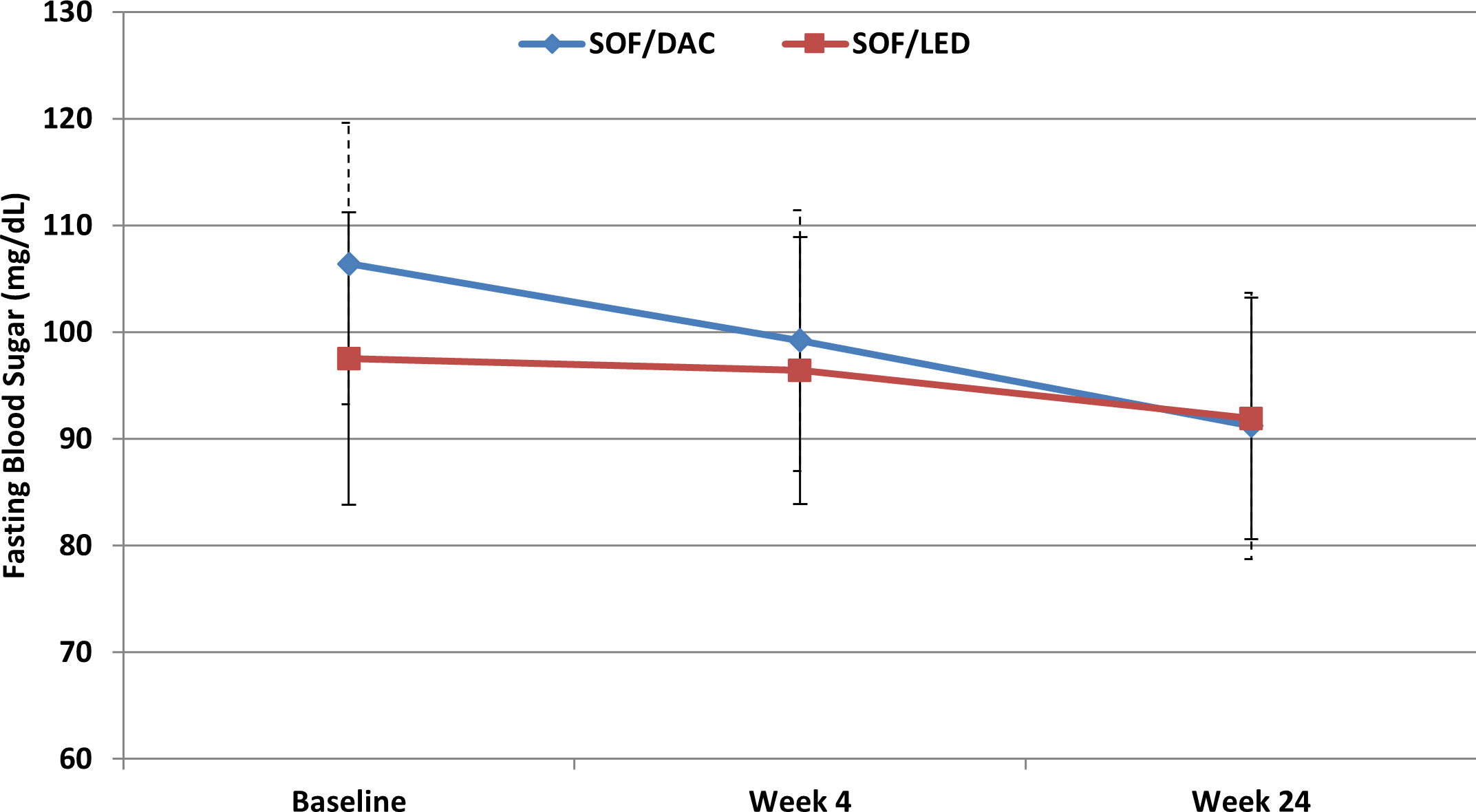
SOF/DAC: sofosbuvir/daclatasvir, SOF/LED: sofosbuvir/ledipasvir, week 4: during treatment, week 24: 12 weeks post end of treatment. Data is expressed as mean±SD. Friedman’s test is used for statistical significance in non-parametric data comparisons with repeated measures. P-value <0.05 is statistically significant.
Chronic HCV treatment has shifted from an IFN-based to an IFN-free regimen combination consisting of direct acting antivirals (DAAS). Twelve weeks of DAA combinations revolutionized the treatment of chronic hepatitis C virus (HCV) due to high sustained virological response (SVR) rates of 95%–100% with tolerable side effects.22,32 Successful treatment using DAAS showed overall improvements in quality of life with decreased mortality due to HCV-related complications, hepatocellular carcinoma, and the need for liver transplantations.22,31,33 Several studies have shown that HCV is associated with impaired metabolic factors such as lipid metabolism and insulin resistance (IR). Successful reversal of chronic of HCV has shown reversal of those metabolic factor alterations.7,9,31 In the present study, significant changes in both lipid profile and glycemic parameters, along with other parameters, were demonstrated in both IFN-free regimens.
Sustained virological response of 100% by quantitative PCR at week 24 was achieved by both groups receiving sofosbuvir/daclatasvir (SOF/DAC) and sofosbuvir/ledipasvir (SOF/LED) regimens in the current study. All 110 patients continued the study without discontinuation due to treatment side effects showing no significant differences between efficacy and safety of both treatment regimens. These results were in concordance with Abdelaty et al. and Nouh et al. where they studied the efficacy and safety of both SOF/DAC and SOF/LED treatment regimens in treatment-naïve genotype-4 chronic HCV Egyptian patients with SVR rates of 98% and 96% respectively and 98% for both groups in the Nouh et al. study with no discontinuation of therapy due to any reported side effects.34,35 Similarly, Essawy et al. achieved 100% SVR in chronic HCV Egyptian patients receiving the SOF/DAC regimen.36
Regarding anthropometric measurements, both groups showed no significant difference in change of body mass index (BMI) after the end of treatment (EOT) when compared to baseline values. These results were similar to Attia et al. who studied the effects of DAAS on glycemic control in HCV diabetic patients; where no difference in body weight or waist circumference were found at EOT when compared to baseline values.37 Also, the results of Allam et al. showed no significant difference in BMI change during the entire study and that obesity had no significant effects on SVR to sofosbuvir-based regimens in chronic HCV Egyptian patients.38
However, Kassas et al. showed an increase in overall BMI in Egyptian patients undertaking several DAAS regimens, including SOF/DAC and SOF/LED, after achieving SVR. The mechanism for increasing BMI with SVR is unclear but they suggested that the increase in BMI might be due to the psychological state improvement which leads to increased patient appetite and taste for food after achieving SVR.39 Non-significant changes of BMI in the results of this study may be due to patients being overweight or obese at the start of the study with baseline BMI values for SOF/DAC and SOF/LED 30.1 ± 6.7 and 25.1 ± 4 respectively.
Successful viral clearance results in liver fibrosis regression due to decrease in hepatic inflammatory markers, which causes deactivation of hepatic stellate cells and myofibroblasts apoptosis.10 A significant decrease in calculated FIB-4 index in the SOF/LED group at EOT compared to baseline values was found, while there were no significant changes in the SOF/DAC group. However, Nouh et al. reported a significant decrease in FIB-4 at SVR 12 with both SOF/DAC and SOF/LED in easy-to-treat patients, while no significant difference was found in FIB-4 in patients receiving the same regimens with advanced liver fibrosis. They concluded that DAAS therapy showed little to no improvement in liver fibrosis in advanced liver disease.35
The interrelationship between host lipid metabolism and HCV was proved by previous clinical studies,21 where HCV uses host cell pathways to produce chronic infection which disrupts the host lipid metabolism.40 Regarding lipid profile, the current study results showed that low-density lipoproteins (LDL) and total cholesterol (TC) levels were increased at week four then decreased at week 24, but at levels higher than baselines levels in both treatment groups. However, comparing the two groups, these changes were more significant in SOF/LED than SOF/DAC. This was in agreement with Mohamed et al. where LDL and TC increased at one month with the SOF/DAC regimen followed by a gradual decrease of lipids at different serial measurements, but at levels higher than baseline.41
Regarding triglycerides (TG) change, SOF/LED showed a significant increase in TG values at SVR 12 compared to baseline (p-value 0.023). However, in the SOF/DAC group, there was a significant increase in TG in week four (p-value = 0.028), which later significantly decreased at week 24 to a level lower than baseline levels.
High-density lipoproteins (HDL) decreased in both groups during the entire study period compared to baseline. However, it was not significant in SOF/LED. This decrease in HDL might be explained by reverse cholesterol transport (RCT) where HDL acts as a transporter of excess cholesterol in peripheral tissue and plasma. HDL carries it to the liver where it can be either metabolized into bile salts to be excreted or directly excreted into bile.42
These results indicate that HCV clearance has a direct effect on host lipid metabolism. This was confirmed by Graf et al. who stated that DAAS are very efficient in eradication of HCV with excellent SVR rates, which leads to increased serum lipid levels during treatment and at SVR. This increase is a reflection of lipid metabolism reversal due to successful HCV eradication.43 Another possible reason for increased lipid levels after HCV clearance could be due to returning of lipids to normal levels pre-HCV infection.20
These findings indicate that successful viral elimination is not the only factor that affects lipid levels during treatment with DAAS, but DAAS themselves may have pharmacological effects on host lipid metabolism. Hence, increases in lipids are strongly dependant on antiviral DAAS type. This is in agreement with Endo et al. and Hashimoto et al. where they found an increase in LDL and TC levels after treatment in both SOF/LED and sofosbuvir/asunaprevir (SOF/ASV) but with a greater increase in the SOF/LED group than the SOF/ASV group. They suggested that DAA type might have different effects on HCV elimination affecting HCV viral kinetics or DAAS themselves may have pharmacological action on serum cholesterol during HCV eradication.22,23 Also, several studies done on Egyptian patients with genotype-4 chronic HCV infection were in agreement with the current study results where the Kamal et al. prospective study found significant increases in TC, TG, and LDL (p-value < 0.001) after treatment with DAAS.44 While El Sagheer et al. found a significant increase in serum LDL and TC at SVR 12 post treatment with sofosbuvir/simeprevir (SOF/SIM).7 In addition, Menesy et al. found a significant increase in serum LDL and TC levels in patients treated with SOF/DAC, SOF/LED, and SOF/SIM regimens, but no significant changes in TG and HDL levels were found.45
Morales et al. did a retrospective study on patients taking SOF/LED, SOF/SIM, and sofosbuvir/ribavirin/interferon (SOF/RBV/IFN) regimens where they found increases in LDL and TC with minimal decrease in HDL irrespective of HCV genotype and HCV antiviral therapy. SOF/LED showed a higher increase in LDL than other SOF regimens; however, it was not significant (p-value = 0.157).9 Moreover, Ozdogan et al. suggested that an increase in LDL and TC is due to direct antiviral inhibition and elimination of DAAS on HCV and that DAAS themselves have no direct effects on lipid metabolism.46
Increases in lipid profiles after treatment with DAAS could be atherogenic, which can predict risk for cardiovascular effects. Hence, it is recommended to long-term monitor lipid profiles after treatment success with DAAS along with cardiological assessment to make sure that lipid increase is simply a pre-infection return of normal lipid levels and ensure that it has no impact on cardiovascular risk.
Regarding glycemic control, this study showed a significant decrease in fasting blood sugar (FBS) and glycated hemoglobin (HbA1c) in both treatment regimens at week four and week 24 compared to baseline in non-diabetic Egyptian patients. An explanation for glycemic control improvement might be due to the direct and indirect effects of HCV on glucose metabolism that promotes IR and inflammatory cascade which increases the risk of developing type-2 diabetes mellitus (T2DM). These alterations are successfully reversed by eradicating HCV with anti-HCV therapy through decreasing systemic inflammation that in return leads to insulin resistance (IR) improvement.47
When comparing both groups, the SOF/LED group had a higher significant drop in HbA1c than the SOF/DAC group. This was in agreement with Morales et al. where they found a higher significant decrease in HbA1c in both diabetic and non-diabetic genotype-1 patients taking SOF/LED when compared to SOF/SIM and SOF/RBV. They suggested that this difference might be due to different DAA interactions with different targets.9
In addition, Jain et al. observed a significant improvement of HbA1c in non-diabetic patients when achieving SVR with the SOF/DAC regimen. They suggested that DAAS are responsible for improvement of glycemic parameters in non-diabetics which in return would prevent the risk of coronary heart disease in patients infected with HCV.31 Also, Adinolfi et al., who included genotype-1 non-diabetic HCV patients, reported that a significant improvement of IR was mainly due to HCV clearance which decreases stress on β-cell and prevents IR-related conditions such as T2DM, cardiovascular disorders, worsening of liver fibrosis, and metabolic syndrome.48
Regarding the effect of different DAA regimens on glycemic parameters, the results of several studies conducted in HCV genotype-4 infected Egyptian patients were in accordance with the results of the current study. All the studies reported significant reductions in FBS and HbA1c after using SOF-based regimens compared to baseline values in either diabetics or non-diabetics who achieved SVR.41,44,47,49
In contrast, other studies didn’t observe a significant change in IR until six months post end of treatment (EOT) and no significant changes in FBS during and at EOT of DAAS were found.43,46,50 Meissner et al. found only a small difference in HbA1c in patients undergoing a SOF/RBV regimen over the period of 24 weeks. However, RBV can have an effect on HbA1c and so it cannot be taken as a marker for glycemic control.21
High BMI is known to be associated with increased IR and hepatic steatosis which can later develop into hepatic fibrosis.47,51 Significant improvement in FBS and HbA1c were reported in the current study even though there were no changes in BMI before and after treatment with DAAS. This suggests that BMI is independent of changes in glycemic control, which supports the role of HCV in IR development. This was in agreement with several studies, where they found no changes or even increases in BMI before and after treatment with DAAS suggesting that improvement in IR is independent of BMI and that it is a consequence of HCV eradication by DAAS.43,47,48,50,52–54 However, Abdel Alem et al. and Attia et al. suggested that improvements in IR and HbA1c are significantly associated with decreased BMI, and recommended lifestyle changes and weight reduction during treatment with DAAS.37,47
HbA1c is a crucial biomarker for long-term determination of glycemic control and plays an important role in diabetic patients’ management. Homeostatic model assessment for insulin resistance (HOMA-IR) is a viable measure of IR. However, HbA1c can be a cheaper practical measure of glycemic control to assess IR by improvements in HbA1c when achieving SVR.9,47 Moreover, the use of HOMA-IR to define IR can be problematic since there are different cut-off insulin resistance levels.3
Patients would benefit from early management of HCV since it can cause early improvements in glycemic control in non-diabetics. This can prevent the future development of IR and T2DM which can alter the course of development of diabetes-related complications.
Our study included a small sample size with a non-cirrhotic non-diabetic study population, which makes our findings not extendable to all HCV-infected population. Moreover, short-term follow-up of both lipid profile and glycemic control after EOT does not allow us to determine if those changes persist beyond the treatment follow-up.
Sofosbuvir-based regimens are highly effective anti-HCV therapy with excellent SVR rates and high tolerability that can improve metabolic and hepatic functions of chronically HCV genotype-4 infected Egyptian patients. It was demonstrated that DAAS had no impact on BMI. However, DAAS resulted in a significant increase in LDL, TC, and TG with decrease in HDL when achieving SVR. Rapid increase in serum lipids during treatment with DAAS is associated with HCV elimination and the type of HCV therapy regimen. Similarly, significant improvement in HbA1c and FBS were also associated with HCV elimination. Moreover, SOF/LED showed higher lipid increases with higher improvements in glycemic control than SOF/DAC.
Figshare: Underlying data for ‘Metabolic changes in chronic hepatitis C patients receiving direct acting antivirals’. https://doi.org/10.6084/m9.figshare.19097063.v125
This project contains the following underlying data:
Figshare: Extended data for ‘Metabolic changes in chronic hepatitis C patients receiving direct acting antivirals’. https://doi.org/10.6084/m9.figshare.19097063.v125
This project contains the following extended data:
Figshare: STROBE checklist for ‘Metabolic changes in chronic hepatitis C patients receiving direct acting antivirals’. https://doi.org/10.6084/m9.figshare.19097063.v125
Data are available under the terms of Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Written informed consent for publication of the patients’ details was obtained from the patients.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hepatology and viral hepatitis
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Sarcopenia and chronic viral hepatitis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Jun 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)