Keywords
Milk, Mastitis, Coagulase Negative Staphylococcus, dairy farm
This article is included in the Pathogens gateway.
Milk, Mastitis, Coagulase Negative Staphylococcus, dairy farm
One of the main challenges of udder health problem of bovine species is mastitis disease which could cause inflammation of cow udders in serious cases (Awale et al., 2012). The disease is peculiar to livestock that is transferred among dairy herds and presently fingered to cause a severe challenge in the dairy sector globally (Gill et al., 2006). A number of micro-organisms of known fungi, bacteria, mycoplasmas, and algae cause mastitis in cows (Batavani et al., 2007). The chief mastitis causing organisms include Streptococcus uberis, Streptococcus dysgalactiae, Staphylococcus aureus, and Streptococcus bovis (Pitkala et al., 2004; Tenhagen et al., 2006). These micro-organisms cause a high bacteria count and low milk quality in lactating cows (Barbano et al., 2006; Oliver, 2012). Dairy cows with mastitis are reported to have low milk production, which in turn reduces market profits of farmers (Halasa et al., 2009; Ampe et al., 2012). The disease is one of the most expensive diseases that affect dairy farms all over the world (Dufour et al., 2012; De Buck et al., 2021). The monetary loss often experienced by farmers due to mastitis disease could be up to an estimated 70 % of all avoidable losses resulting from milk production (Sumathi et al., 2008). This review is based on a dissertation submitted by the author in partial fulfilment of the requirement for a Master of Science (MSc) in Animal Science (Idamokoro, 2013).
Staphylococcus species are known bacteria that are commonly identified in milk samples of most cows infected with clinical and subclinical mastitis (Pitkala et al., 2004; Tenhagen et al., 2006). They are classified as coagulase-positive staphylococci (CPS) and coagulase-negative staphylococci (CNS) and have been linked with the cause of subclinical and clinical mastitis (De Vliegher et al., 2003; Taponen et al., 2006; De Buck et al., 2021). The regular species of CNS include Staphylococcus Chromogenes, Staphylococcus xylosus, Staphylococcus epidermidis, Staphylococus hyicus, Staphylococcus simulans, and Staphylococcus haemolyticus (Thorberg et al., 2009) while examples of CPS comprise of Staphylococcus aureus and Staphylococcus hyicus (Awale et al., 2012). Of late, CNS has emerged in dairy farms as a mastitis causing organism (Simojoki et al., 2011). CNS is becoming a significant mastitis causing organism in several nations including developing countries (Petzer et al., 2009; Sender et al., 2017). These micro-organisms are frequently isolated from the cow udder duct, udder teats, skin, and milk of cows that have signs of clinical and subclinical symptoms (De Vliegher et al., 2003; Taponen et al., 2006).
CNS are commonly treated as a group even though they differ in both their phenotypic and genotypic traits (Layer et al., 2007; Onni et al., 2010). For easy identification of the various species of CNS, numerous methods including analytical profile index (API staph 32 ID) and the Staph Zym test have been adopted. The prevalence at the species level is necessary in order to ascertain the impact that they play in causing mastitis in dairy farms (Pyorala and Taponen 2009). This manuscript seeks to address the trending emergence of CNS in relation with their prevalence, pathogenicity, global incidence indices in bovine milk and some possible panacea for curbing its spread in dairy farms.
The topic of mastitis is of a great concern despite strategies put in place to curb the prevalence in dairy farms. The resultant cause of mastitis infection has led to poor performance of cows in terms of milk quality and milk production, and increased cost of treatment of affected animals (Hunderra et al., 2005). Mastitis is reported to be subclinical in most instances and it results into udder health problems. Lately, CNS has been revealed to cause subclinical mastitis and at other instances they cause mild form of clinical mastitis (Taponen et al., 2009). Their contribution in instigating udder health problems is a worry to farmers, researchers, and veterinarians globally. With these concerns, it may be safe to say that more research on tackling the threat that they may pose to the dairy industry be adequately addressed. Figure 1 gives a summary of the multi-facet dimensions of CNS mastitis in relation to issues of its infections in dairy farms.
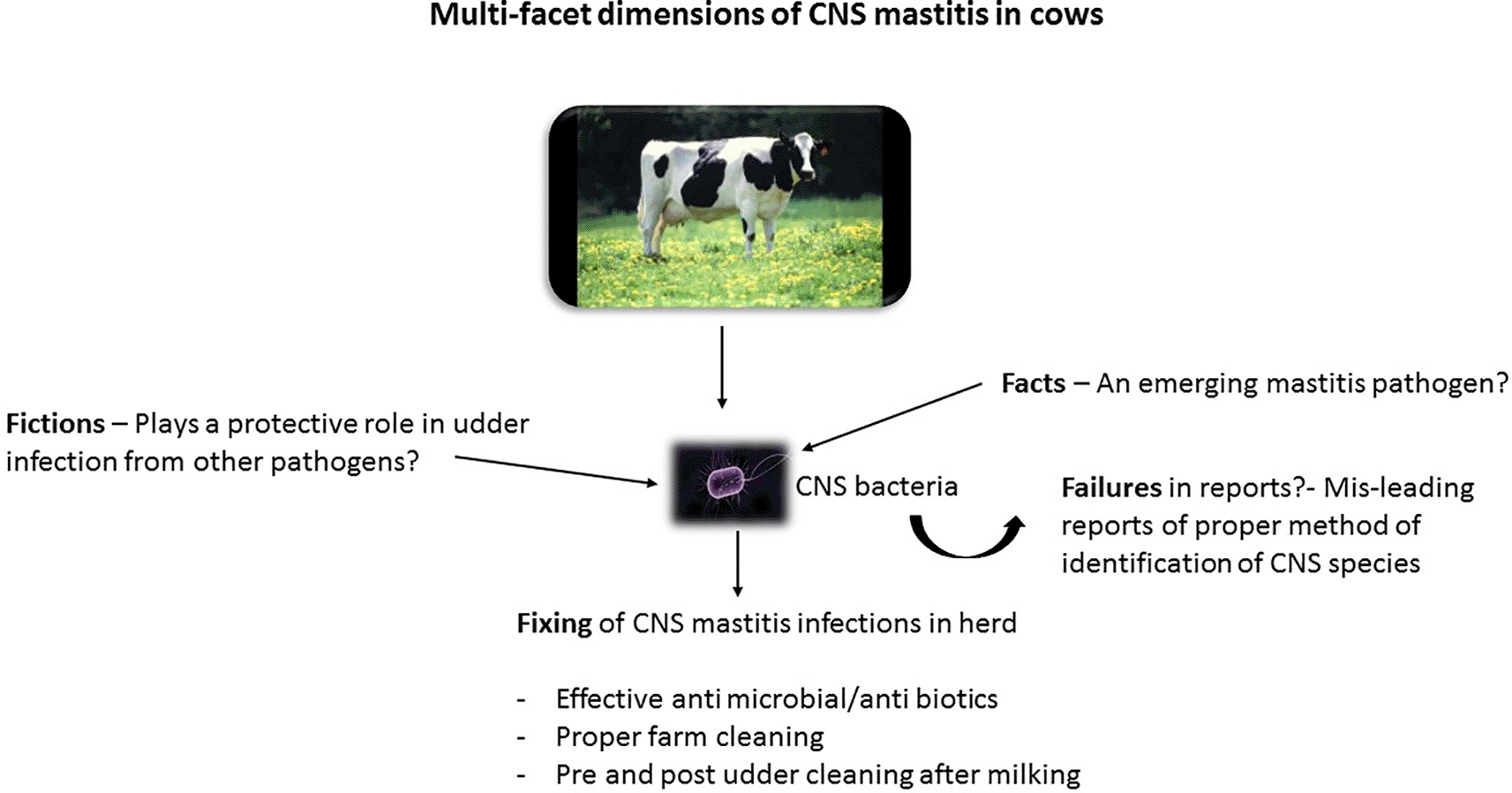
Some pathogenic bacteria are the main cause of mastitis. Others are mentioned as minor pathogens as a result of the fact that they have insignificant or no impact in causing mastitis in cows. Among the pathogenic bacteria previously classified as minor pathogen by scientists is CNS. However, in recent times, several reports have shown their involvement in mastitis infection (Pitkala et al., 2004; Rajala-Schultz et al., 2004). Among these CNS mastitis-causing organisms are Staphylococcus epidermidis, Staphylococcus simulans, Staphylococcus chromogenes, Staphylococcus xylosus, and Staphylococcus haemolyticus (Aarestrup et al., 1999; Taponen et al., 2006). Some identified chief mastitis pathogens are Streptococcus dysgalactiae, Escherichia coli, Streptococci agalactiae, Staphylococcus aureus, and Streptococcus uberius (Peterrson-Wolfe et al., 2010; Philip et al., 2011; Schmidt et al., 2015). The incidence and prevalence of mastitis causing agents have been associated with several factors such as cow health, management practices, cow immunity, mammary gland physiology, and dairy farm environment (Waage et al., 2000; Zadoks et al., 2002; Oviedo-Boyso et al., 2007).
Staphylococcus species is a niche of bacteria that is grouped according to their capability to coagulate blood plasma. They are classified as CNS on one hand and CPS on the other hand. Overall, an aggregate of 50 Staphylococcus species and subspecies has been categorized and identified from this group (Pyorala and Taponen, 2009). Cow udders become very red and swollen in severe cases due to of infection resulting from bacterial pathogens that are linked with mastitis (Dufour et al., 2012). Whereas CNS is noted to be a pathogen of less economic significance in bovine mastitis, their role in intra mammary infection leading to mastitis in cows is still very much relevant (Pyorala and Taponen, 2009). The transfer of mastitis infection through the mammary gland from one cow to another is caused mostly by bacteria such as CNS seen in subclinical mastitis (Djabri et al., 2002; Nyman et al., 2018). The isolation of CNS from bovines with clinical and subclinical mastitis has been well reported (Jorun, 1991; Myllvs, 1995; Tenhagen et al., 2006; Koivula et al., 2007). Suffice to say is that, the two predominant CNS species causing mastitis are Staphylococcus chromogenes and Staphylococcus simulans. These two most known CNS affects dairy cows at varied parities and at different lactation stages.
The ability of CNS to cause mastitis hints that they should be researched on as an important mastitis causing agent and not merely as minor pathogens of bovine skin micro flora (Taponen et al., 2009). Over the years, researchers have yet to come to terms on the right definition for intra mammary infection resulting from various bacteria in cows due to mastitis (Andersen et al., 2010). Information on the effect of CNS in relation to mastitis and human health is however rare (Thorberg, 2008; Osman et al., 2015). This has put the issue of redressing the role of CNS as a mastitis causing organism on researchers’ spotlight.
The infections of cows caused by CNS and their capability to cause mastitis often happens at different lactation stages. Cows that have calved only once are reported to be infected with CNS at the early lactation stage while multiparous cows are infected at the late lactation period (Grohn et al., 2004; Taponen et al., 2007). Species of CNS that infect heifers differ from the ones that infect cows; because bovine infections caused by CNS are age dependent (Pyorala and Taponen, 2009). The regularly isolated mastitis causing CNS species are Staphylococcus chromogenes, Staphylococcus xylosus, Staphylococcus haemolyticus, Staphylococcus epidermidis, Staphylococcus simulans, and Staphylococcus hyicus (Trinidad et al., 1990; Matthews et al., 1992; Thorberg et al., 2006; Frey et al., 2013). Persistent CNS intra mammary infection (Leroy et al., 2015) may linger for a long time during lactation, when milking cows are detected and treated late by dairy farmers (Aarestrup et al., 1999; Oliver et al., 2003; Gillespie et al., 2009).
The spread and prevalence of mastitis caused by CNS varies from one country to another (Table 1). Season dynamics and weather changes also add to the proportion of CNS pathogens that results in mastitis spread among herds (Makovec and Ruegg, 2003; Osteras et al., 2006). Furthermore, the type of housing system, parity, lactation stage, variation in sampling techniques, level of farm production, and method of species identification are other factors that influence the spread and prevalence of mastitis caused by Staphylococcus species (Thorberg, 2008). Often times, CNS occurs more in subclinical mastitis when compared to those of clinical mastitis (Pyorala and Taponen, 2009). Lack of sensitization of farmers on the potential threat of CNS to cause mastitis infection may be reason for mastitis problems, and information on the prevalence of CNS is useful in the control of mastitis spread caused by CNS (Pyorala and Taponen, 2009).
| Nation of origin | Percentage (%) | Source |
|---|---|---|
| Canada and USA | 15 | Dingwell et al. (2004) |
| Estonia | 16 | Haltia et al. (2006) |
| Finland | 24-50 | Pitkala et al. (2004); Koivula et al. (2007) |
| France | 13.7 | Botrel et al. (2010) |
| Germany | 35 | Tenhagen et al. (2006) |
| Netherland | 6 | Poelarends et al. (2001) |
| Norway | 16 | Osteras et al. (2006) |
| South Africa | 61 | Petzer et al. (2009) |
| USA (Tennessee) | 28 (Herds with high SCC) 12-41 (Herd prevalence) | Roberson et al. (2006) |
Isolation of CNS from cows with clinical mastitis has also been reported in some countries (Table 2) and seasons is a factor that determines the prevalence of CNS mastitis in different countries. A study by Koivula et al. (2007) in Finland and Osteras et al. (2006) in Norway revealed that there is seasonal influence on the prevalence of CNS mastitis in bovines. With regards to intra mammary infection in dairy herds, the prevalence of CNS is higher in heifers when compared with older cows (Sampimon et al., 2009a). The understanding of CNS spread within certain areas and their species-specific prevalence will help to fight the issue of mastitis related to CNS.
| Nation of origin | Percentage (%) | References |
|---|---|---|
| Finland | 18 | Koivula et al. (2007) |
| Israel | 9 | Shpigel et al. (1998) |
| Wisconsin (USA) | 17.5 | Makovec and Ruegg (2003) |
| Poland | 14.6 | Malinowski et al. (2006) |
In the dairy sector the issue of mastitis incidence in herds is one of the criteria and parameters utilized for determining udder health (Olde-Riekerink et al., 2007). Specific cow and bulk tank somatic cell counts are other parameters used to detect udder health problem (Olde-Riekerink et al., 2007). However, the incidence level of mastitis is influenced by season (Morse et al., 1988; Olde-Riekerink et al., 2007). Cows are subjected to high incidences of clinical mastitis in the fall (December) than in summer (Olde-Riekerink et al., 2007). Inter-play of specific mastitis disease causing organisms in cows with regards to seasons likewise exist (Osteras et al., 2006). A higher prevalence of CNS and Streptococcus dysgalactiae was declared in winter than in other seasons (Osteras et al., 2006). Hogan et al. (1989) and Makovec and Ruegg (2003), in their studies also reported that the incidence rate of mastitis was higher for streptococci and coliforms in summer. In addition, mastitis prevalence caused by Staphylococcus aureus and Streptococcus uberis increased in summer compared to other seasons (Osteras et al., 2006).
Recent studies show that some CNS species display inhibitory traits against key mastitis causing pathogens that may want to result into intra mammary infection in dairy cows thereby acting as a protection to cow teats and udders (Reyher et al., 2012a). For instance, Staphylococcus chromogenes is a type of CNS species that represses other important mastitis causing organisms from infecting the udder of bovines (De Vliegher et al., 2004). There are contradictory reports as to whether CNS actually gets involved in a protective role or promotes the risk of infection on udder quarter of cows (Green et al., 2002; Reyher et al., 2012b). According to Zadoks et al. (2001), CNS species do not display a protective influence against the udder quarter of cows and they also do not expose them to risk of infection by major mastitis pathogens. Matthews et al. (1990) and Lam et al. (1997) in their study reported that cow quarters infected with CNS are likely to show resistance to any later infections by Streptococci species and Staphylococcus aureus.
Conversely, an in-vitro study conducted by De Vliegher et al. (2004) revealed the inhibition of Streptococcus uberis, Staphylococcus aureus, and Streptococcus dysgalactiae by Staphylococcus chromogenes. Dos Santos Nascimento et al. (2005) in their study likewise revealed the inhibition of Staphylococcus agalactiae, which is a major mastitis pathogen by some strains of CNS. The secretion of antibacterial peptides by CNS species is a likely mechanism used in the inhibition of major mastitis (Dos Santos Nascimento et al., 2005; Sawant et al., 2009). In the study by De Vliegher et al. (2004), Staphylococcus chromogenes secreted substances that repressed the growth of Staphylococcus aureus and other streptococci. Contradicting studies by Compton et al. (2007) and Parker et al. (2007) reported that CNS do not show any protective effect on udder quarters of cows against major mastitis. In order to properly understand the protective and risk factor effect of CNS bacteria on udder quarter of cows factors such as age, lactation stage, immunity level, and anatomy of cows are to be considered (Reyher et al., 2012b). With various contradictory reports from studies on the actual role played by CNS in protecting cow udder or their ability to cause mastitis, scientist should be cautious on their claims (giving empirical facts) when reporting to the global scientific world of the actual role of CNS.
There is high urge to increase individual cow performance and productivity. As a result of this, farmers have become more dependent on antibiotics (McKenna, 2011) as they are utilized for the treatment and prevention of bovine metritis and mastitis (Walther et al., 2008). They are also used for improving growth in livestock, as therapeutics and prophylactics agents (Sawant et al., 2005). Some of the antibiotics used in farms include penicillin, oxacillin, tobramycin, ciprofloxacin, tetracycline, erythromycin, cefazolin, clindamycin, and beta lactams (Sawant et al., 2005; Gao et al., 2012). Studies reveal certain variations in resistance to antibiotics by different CNS at their species level (Luthje and Schwardz, 2006; Waller et al., 2011; Saini et al., 2012; Dorneles et al., 2018). Sawant et al. (2009) in their study, reported that Staphylococcus epidermidis was resistant against pirlimycin, erythromycin, and methicillin but other CNS species including Staphylococcus hyicus, Staphylococcus simulans, and Staphylococcus chromogenes were susceptible to them. Resistance against ampicillin by Staphylococcus hyicus, Staphylococcus epidermidis, and Staphylococcus chromogenes was also indicated by Sawant et al. (2009). Pitkala et al. (2004) also reported the resistance pattern of some antibiotics for Staphylococcus aureus and CNS including erythromycin, streptomycin, gentamycin, and oxacillin was between 0-5.1% and 0-9.96% respectively. In that study, resistance to penicillin was high for Staphylococcus aureus and CNS (52.1% and 32% respectively).
The prevalence of antimicrobial resistant CNS bacteria such as Staphylococcus haemolyticus, Staphylococcus chromogenes, Staphylococcus epidermidis, and Staphylococcus simulans against penicillin was 70%, 33%, 18%, and 0%, respectively (Sampimon, 2009). Resistance against oxacillin, tetracycline, sulphonamides, trimethoprim, clindamycin, methicillin, and kanamycin by CNS has also been indicated (Gentilini et al., 2002; Moon et al., 2007; Bengtsson et al., 2009; Frey et al., 2013). Apart from dairy cows (Raspanti et al., 2016), other farm animals have been pointed to show resistance to antibiotics by CNS. Lollai et al. (2008) revealed resistance in isolates of milk samples retrieved from sheep against some antibiotics by CNS. Resistance to penicillin in sheep is lesser than that of cattle (Lollai et al., 2008). Factors including incidence of disease, level of education, cost of treatment, type of antibiotics, and farm management practices influence the use of antibiotics for treatment of mastitis (Grave et al., 1999; Sawant et al., 2005). The continual utilization of antibiotics in livestock farming has resulted in the increase in resistance to several antibiotics by bacteria. Regularly monitoring antimicrobial profiles in livestock is very vital to collect data for trends/emergence in antimicrobial resistance genotypes and phenotypes to recognize and identify new or emerging resistance profiles (Crespi et al., 2022).
The impact CNS species play in infecting cow udders cannot be over emphasized. Despite the fact that antibiotics have been used to manage and treat cows that show mastitis and persistence intra mammary infections caused by CNS species (Taponen et al., 2006; Simojoki et al., 2009), treatment has always been carried out in clusters as a group. Recently it was reported that different species of CNS exhibit different virulence characteristics (Zhang and Maddox, 2000; Waller et al., 2011). There are variations in the virulence determinates of CNS. Often times CNS are not identified at the species level but as a group. The reason for this is because the diagnostic significance of CNS species in dairy maintenance has not yet been rightly looked into (Zadoks and Watts, 2009).
It is important to recognize mastitis pathogens at the species level including CNS (Rossitto et al., 2002; Pitkala et al., 2004). Pathogens that cause mastitis have varied levels of pathogenicity and virulence characteristics (Zhang and Maddox, 2000; Waller et al., 2011). The severity of infection caused by Staphylococcus chromogenes in livestock is much higher than other CNS (Lasagno et al., 2018). Staphylococcus chromogenes, which have been indicated as a minor mastitis pathogen, can cause severe damage to cow udders when compared to Staphylococcus aureus (Myllys et al., 1994). Additional studies and research on species-specific differences as related to their virulence traits will assist in effective mastitis control management in dairy farm.
CNS are a collection of Staphylococcus species commonly isolated from the milk of cows with clinical subclinical mastitis and microbes-free clean milk. They are reported to be pathogens that are becoming dominant in the cause of cow mastitis. About 39 various species of CNS have been classified at the species level. The recognition and identification of CNS in most cases is treated as a collection/group and not as individual species. It was recently revealed that different species of CNS display different virulence traits (Zhang and Maddox, 2000; Waller et al., 2011). Identifying CNS as a group may not be enough when considering effective therapy and mastitis control program but identifying them at the species level using more precise methods (such as gene sequencing) may suffice (Lange et al., 2015).
The standard identification process for CNS at the species level is the utilization of conventional biochemical methods. This includes growth of species in various prepared media, morphology of species in colonies, gram staining, catalase production, and coagulase tests among others. The use of conventional biochemical tests is more demanding, expensive, and time consuming (Couto et al., 2001). Plenty of the commercial biochemical kits have been manufactured to identify CNS at the species level phenotypically. The Staph-zym system, ID 32 staph test, and ATB 32 staph differentiation system are some commercial biochemical test kits that have been utilized to identify CNS at the species level (Deinhofer and Pernthaner, 1995; Thorberg and Brandstrom, 2000; Capurro et al., 2009). The use of ID 32 staph test for identifying CNS is efficient and also cheap when compared to the staph-zym test (Thorberg and Brandstrom, 2000; Sampimon et al., 2009b). Conversely, there is limitation in the utilization of commercial biochemical kits for identification of all species of CNS from animal source (Bes et al., 2000). The use of molecular test procedures for identifying CNS species still remains the best till date (Zadoks and Watts, 2009).
CNS also demonstrate a threat to the cow udder in a poorly managed milking parlour and farm environment (Hunderra et al., 2005; Philip et al., 2011). These CNS pathogens are very contagious and can persist in the udder and the cow teat canal of infected cows hereby increasing the risk of infection to healthy cows during milking (Peterrson-Wolfe et al., 2010; Mahmmod et al., 2018). Proper monitoring and management practices and hygiene in the milking parlour are important in avoiding the widespread of microbes and bacteria when cows are been milked (Yuen et al., 2012). The risk of infection for contagious and environmental mastitis pathogens including CNS becomes much higher when milking machines are ineffectively managed (Kim et al., 2019). Unethical practices in the milking parlour can also expose cows to mastitis causing organisms. There are certain practices such as using the same towel to clean cows’ udders or improper drying of cow teats during milking of cows will proliferate the distribution and spread of mastitis causing organisms (Jones, 2010). More awareness on the need to curb any potential threat that may lead to infections by CNS should be created among dairy farm workers. This can be achieved by sensitizing dairy farm workers’ of the persistent nature of CNS on the surrounding of cow udders and the milking environment.
Effective cleaning of cow teats with treated chemicals and disinfectants before milking, proper hygiene, culling of chronically infected cows, dipping of teats with chemicals, and effective cow therapy are practices employed to reduce the load of mastitis causing organisms around cow udders during milking (Barkema et al., 2006). Notably, before any control measures for the prevention of CNS mastitis can be launched, more information is required about the CNS species linked with mastitis and the form and virulence of the diverse species. The importance of effective anti-biotic strategy treatment, good hygiene, and cleaning management in the milking parlour to curb the spread of CNS mastitis in cows cannot be over-emphasized (Kim et al., 2019).
In a proper dairy farm according to the Food standards Agency Scotland (2007), effective hand washing prior and during milking activities, wearing of clean clothes by milking operators, and the engagement of physically healthy milking workers should be employed when milking cows. This helps to curb the possible spread of mastitis causing organisms from one cow to another and also prevent milk from bacterial contamination.
Some CNS strains isolated from mastitis may be opportunists from the milking surrounding. However, according to Pyorala and Taponen (2009) in their account, it was opined that it is very likely that at least the major species infecting dairy cow mammary glands are precisely adjusted to the udder surroundings. Species of CNS may differ in this respect, however, scientific proof is lacking. Furthermore, in most dairy herds, pregnant heifers are more likely to be infected with CNS than cows. In solving the threatening challenges of CNS mastitis causing pathogens, more focus may therefore be on the heifers, i.e., their environment, feeding, and management, before calving. Conversely, it could be noted that the welfare and comfort of heifers may be momentous factors for good udder health.
The wearing of hand gloves by workers during milking procedures helps to reduce intra mammary infection of Staphylococcus aureus (Dufour et al., 2012). Frequent cleaning and sanitation of milking machines, correct cleaning of vacuum systems, clean milking environment, and the correct utilization of cleaners and sanitizer during cow milking prevents bacteria contamination of raw milk (NIANR, 2006). Fore stripping of cow milk and frequent screening of fore milk prior to attaching milking clusters to teats are also efficient measures carried out to curb the spread of bacteria during milking operations (Oliver, 2012).
It is not out of place for the staphylococcal mastitis strains including CNS to be transferred between humans and dairy cows (Sender et al., 2017). However, the transmission of these non-aureus mastitis causing organisms is very possible (Sakwinska et al., 2011). In the study by Thorberg et al. (2006), it was reported that the same strains of CNS (S. epidermidis) on milkers’ hands was also found in milk and it was suspected that milking personnel are the source or carrier of the bacteria to dairy cows. CNS usually accompanies infections with main pathogens that are treated with antimicrobial drugs (Sender et al., 2017). The assumptions by some researchers that CNS harbours drug resistance genes for other bacteria, including E. coli, means that the Streptococcus species is very worrisome (Frey et al., 2013; Ottom, 2013).
At present, studies on CNS species as a mastitis causing organism in dairy cows is scarce. Judging from the current knowledge, it may be hard to ascertain whether CNS species act as contagious or ecological pathogens. However, the pathological contribution of CNS in bovine mastitis has inevitably surged and there is an urgent need to pin-point the threat to both human and animal health, originating from this micro-organism. Effective management procedures against contagious mastitis organisms, such as pre- and post-milking teat disinfection, may assist in lowering CNS infections in cows during milking in dairy farms. Appropriate methods such as molecular identification of CNS species rather than phenotypic method of identification may be more reliable in identifying CNS in the milk of mastitis cows for proper management strategies. Unarguably, it may be more beneficial to the dairy industries if more research on the epidemiology of CNS causing mastitis in dairy milk and more reliable methods for species identification is carried out by scientists for proper farm management purpose.
We appreciate the moral and logistic support from Dr. Y.S. Hosu for towards writing of this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Veterinary Microbiology and Milk Quality
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 25 Jul 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)