Keywords
behavioural activation, adapted intervention, diabetes, depression, multimorbidity, feasibility trial protocol, South Asia, Low and Middle-Income countries
behavioural activation, adapted intervention, diabetes, depression, multimorbidity, feasibility trial protocol, South Asia, Low and Middle-Income countries
Type 2 diabetes (T2DM) is one of the most common non-communicable diseases worldwide. It currently affects approximately 536.6 million people worldwide, with numbers expected to reach 624.7 million by 2030.1 The burden of T2DM is disproportionately higher in Low and Middle-Income Countries (LMICs) - which account for up to 80% of the global diabetic population.2 A particular case is the densely populated South Asian region; in countries like Bangladesh and Pakistan, the estimated prevalence of T2DM stands at 11.4% and 16.7% respectively, which is higher than the global average.3,4
A number of studies have previously demonstrated that T2DM is associated with a higher risk of developing depression.5,6 T2DM and depression multimorbidity is associated with increased healthcare expenditure7 and poorer health and clinical outcomes - the latter include, but are not limited to; deterioration in self-management practices, glycemic control, deterioration of quality of life, the onset of complications8–10 and mortality.11–13 In LMICs, the lack of accessible and affordable treatment continues to expand the treatment gap for mental health problems, compounding health outcomes for those with multimorbidity. Evidence-based and cost-effective interventions are therefore urgently needed in these high burden, low resource settings to ensure health and sustainable development.
Behavioural activation (BA), is simple and effective psychotherapy used for the treatment of depression encouraging individuals to reconnect with valued activities and positive reinforcement in their environment.14–18 BA conceptualizes depression as an interaction of a person with their environment that may be less stigmatizing when compared to other narratives of depression.19 BA has a straightforward, stepped activity scheduling approach16–21 and requires minimal resources compared to other psychotherapies as it can be effectively and easily administered by mental health staff or non-mental health specialists.22,23 BA has been adapted for different cultures and populations,24 however there is limited evidence of its effectiveness for treating depression in patients with chronic physical illnesses such as diabetes and particularly in LMICs.
A recent Cochrane review assessing the efficacy and acceptability of BA for treating depression in Non-Communicable Diseases (NCDs) identified two studies from the United States, one for stroke and one for breast cancer, but the results were inconclusive.25–27 Another trial conducted in India demonstrated a reduction of depressive symptoms, but this included BA strategies within a multicomponent intervention.28 There is a need to generate evidence on the effectiveness and cost-effectiveness of BA interventions for treating depression in LMIC populations with T2DM. Before embarking on such a trial, we first need to establish the feasibility and acceptability of BA adapted for cultural context and for people with T2DM and the feasibility of trial procedures. The objectives of this study, therefore, are to:
We will conduct a multi-country, parallel-arm, randomised-controlled feasibility trial of the DiaDeM intervention versus optimised usual care, along with embedded economic and mixed methods process evaluations. The study population are adult outpatients in Bangladesh and Pakistan attending specific health facilities with established diabetes services, having a confirmed diagnosis of T2DM and mild, moderate or severe depression. The methods for the proposed feasibility trial are reported, in line with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)29,30,73 and Template for Intervention Description and Replication (TIDieR)31,73 guidelines.
The DiaDeM feasibility trial will be conducted in Bangladesh and Pakistan. Study sites will be the following six secondary and tertiary health care facilities with established diabetes services:
1. BIRDEM General Hospital (Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders), Dhaka, Bangladesh,
2. Sylhet Diabetic Hospital, Sylhet, Bangladesh,
3. Sugar Hospital, Phase V, Hayatabad, Peshawar, Khyber Pakhtunkhwa, Pakistan,
4. District Headquarter Hospital, Kohat, Khyber Pakhtunkhwa, Pakistan,
5. Sughra Diabetic Centre, Benazir Bhutto Hospital, Rawalpindi, Punjab, Pakistan, and
6. District Headquarters hospital, Rawalpindi, Punjab, Pakistan.
The sites have been selected based on the availability of non-mental health specialist staff in diabetic services, geographically located in different provinces, with extensive catchment areas allowing diverse patient population availability.
T2DM status will be confirmed by the health care staff of the diabetes centres, based on standardized diagnostic criteria (clinical presentation and glycemic levels measured using HbA1c) and diabetes centres’ registration records. Depression status will be established through a two-stage screening process. Patient Health Questionnaire-2 (PHQ-2)32 will be administered by the health care staff at the diabetes centres incorporating it into routine clinical practice, and study information leaflets provided to all those screening positive (PHQ-2 ≥3). Those indicating interest in participating will be approached by a research assistant (RA), who after taking written informed consent for assessment for depression, will administer the 9-item depression module of Patient Health Questionnaire-9 (PHQ-9).33,34 Those with a score of PHQ-9≥ 5, will be categorized to have mild, moderate or severe depression confirmed by clinically trained researchers using the depression schedule of the Mini International Neuropsychiatric Interview (MINI) scale (version 6.0).35 The MINI is a short diagnostic interview designed to allow administration by non-mental health specialists36 which has been validated in both Bengali and Urdu versions.37
Patients already receiving psychotherapy for depression or lacking the capacity to provide informed consent and/or to take part in therapy because of cognitive impairment, or severity of mental or physical illness will be excluded from the study.
A unique screening number will be assigned to every individual screened and will be entered into a screening log. Screened, eligible participants will receive a study information sheet written in local languages and complying with local ethics requirements. Participants will be informed that they can withdraw consent and leave the trial at any point in time, without giving a reason and by informing any of the research team or the staff at the diabetes centre. Those willing to participate will be provided hard copies of consent forms to read and record their signatures; see Extended data73 for a copy of this form. Those unable to provide a signature will be requested to record consent using their thumbprint on the same paper copies.
Eligible and consenting individuals will be assigned a unique patient identifier (ID). They will then be randomly assigned to DiaDeM or optimised usual care by RAs. This will be done using a computer-generated block randomisation sequence stratified by country created using Stata V16 (StataCorp, 4905 Lakeway Drive, College Station, Texas, 77845 USA), with an allocation ratio of 1:1. A statistician based at the University of York, who is not involved in the recruitment of trial participants, will generate the randomisation sequence. An online randomization system will be developed to ensure allocation concealment. Allocation will be recorded on the participant record.
This is an assessor-blinded trial. The trial manager will set up a log of the participants recruited and the RAs responsible for baseline and follow-up assessments. It will be strictly ensured that the RAs who will recruit, randomise and allocate a participant to the trial will only complete the baseline assessment for that patient, with three months and six months of follow-up Case Report Forms (CRFs) of the study participants completed by another RA based at the same study site.
The sample size was based on estimating recruitment and follow up rates to within a margin of error. We will recruit a total of 128 participants, 64 each in Bangladesh and Pakistan to allow for the estimation of recruitment (50%) and follow-up rates (80%) to within a 9% and 10% margin of error.
DiaDeM arm
Participants randomised to the DiaDeM arm will receive a culturally adapted BA intervention (DiaDeM) in addition to the ‘optimised usual care’ leaflet, details of which are provided in the next subsection.
The DiaDeM intervention is a culturally adapted BA, modified using the input of persons with diabetes and depression multimorbidity, caregivers, healthcare professionals, policymakers and an expert panel. The intervention adaptation was guided by the five steps of the Intervention mapping framework for the development, implementation and evaluation of health promotion interventions38 and the Bernal39 and the Escoffrey40 frameworks to deal with the cultural and context components of the adaptation process. The Stirman adaptation classification41 was used to map changes made to the original intervention i.e. the BA for Multimorbidity in older adults intervention (MODS) programme. The adaptation process has been documented and will be published in the near future.
DiaDeM BA targets unhelpful avoidant behaviours that negatively reinforce the cycle of depression, replace them with positively reinforcing behaviours to re-establish their usual routines, increase their opportunities to experience pleasure and break the cycle of depression and promote self-management of diabetes. DiaDeM will be delivered by non-mental health specialist staff titled BA facilitators, based in diabetes services (as diabetic educators, diabetic nurses, nutritionists, paramedics etc.) after receiving formal training by the BA experts. The individual sessions will be delivered by BA facilitators, using a treatment manual and supporting intervention materials (participant booklet, BA facilitator logs, supervisor checklists) with supervision from a mental health specialist (BA supervisors).
Participants receiving the DiaDeM intervention will have six individual BA sessions of 30-40 minutes each, either face-to-face or remotely over a period of 6-12 weeks, at the diabetes centres. The sessions will be booked weekly but will allow flexibility for the participants who wish to match the session timings with their diabetes consultations or in case there are any depression/diabetes complications.
Control arm
Participants randomised to the control arm will continue to receive diabetes care from the same facility. In addition, they will also receive an ‘optimised usual care’ leaflet, describing depression and its treatment with details on how to access help. The referral and advice to the control arm will be provided by non-mental health specialist staff working at each facility. Participants in this trial arm will not have any further contact with the non-mental health specialist for study purposes.
Following baseline data collection and randomisation, participants in each arm will be followed up at three and six months (Table 1). At baseline (in addition to demographics and socioeconomic status) and at six-month follow-up, we will record weight, height, Body Mass Index (BMI), waist circumference, hip circumference, waist-hip ratio, smoking status, blood pressure, comorbidities, depression caseness and severity,33 anxiety,42 diabetes distress,43,44 self-efficacy,45,46 diabetes self-management activities,47 physical activity,48 diabetes microvascular and macrovascular complications, medication and health-related quality of life.49,50 We will also investigate blood tests including glycosylated Haemoglobin (HbA1c), Haemoglobin level (Hb) and White Blood Cell count (WBC), renal function tests including serum urea, serum creatinine and estimated Glomerular Filtration Rate (eGFR), lipid function test including triglycerides, total cholesterol, High-Density Lipoprotein cholesterol (HDL), Low-Density Lipoprotein cholesterol (LDL), thyroid function tests including free serum Triiodothyronine (T3), free Thyroxine test (T4), Thyroid-Stimulating Hormone (TSH), Random Blood Sugar (RBS), and Liver function test including only serum Alanine Transaminase (ALT).
| Trial outcomes | Scales or tools for data collection | Base-line | Month 3 | Month 6 |
|---|---|---|---|---|
| Demographics | Adapted from WHO STEPwise approach to Surveillance (STEPs) instrument*, Version 3.2 | X** | ||
| Physical body measurements (Weight, height, Body Mass Index, waist circumference, hip circumference, waist-hip ratio, and blood pressure) | WHO STEPS surveillance* manual for measurements | X | X | |
| Blood tests: Haemoglobin level (Hb), White Blood Cell count (WBC), renal function tests including serum urea, serum creatinine and estimated Glomerular Filtration Rate (eGFR), lipid function test including triglycerides, total cholesterol, high-Density Lipoprotein cholesterol (HDL), low-Density Lipoprotein cholesterol (LDL), thyroid function tests including (serum free Triiodothyronine (T3), free Thyroxine test (T4), Thyroid-Stimulating Hormone (TSH), Random Blood Sugar (RBS), and serum Alanine Transaminase (ALT). | Blood test checklist | X | ||
| Blood test: Glycosylated haemoglobin (HbA1c) | Blood test checklist | X | X | X |
| Comorbidities | Adapted from the STEPs module* for non-communicable diseases and communicable/infectious diseases | X | X | |
| Caseness and severity of depression | Physical health questionnaire (PHQ-9) | X | X | X |
| Anxiety | Generalized Depression and Anxiety (GAD-7) | X | X | |
| Diabetes distress | Problem Areas in Diabetes Scale-5 (PAID-5) | X | X | |
| Self-efficacy | Diabetes Empowerment Scale-Short form (DES-SF) | X | X | |
| Diabetes self-management and self-care | Perceived Diabetes Self-Management Scale (PDSMS) Summary of self-care diabetes activities scale (SDSCA) | X | X | |
| Health risk behaviour: Physical activity | International Physical Activity Questionnaires (IPAQ, short version) | X | X | |
| Health risk behaviour: Tobacco use | An adapted version of The STEPs tobacco and smokeless tobacco modules. | X | X | |
| Health risk behaviour: Alcohol use | Adapted questions of the STEPs alcohol module. | X | X | |
| Diabetes complications | A set of questions in DiaDeM CRF on Diabetes-related microvascular and macrovascular complications. | X | X | |
| Health-related quality of life (HRQoL) | EQ 5D-5L including visual analogue scale (EQ-5d-VAS) | X | X | X |
| Mediators | A set of questions in DiaDeM-CRF for potential mediators including; Knowledge about depression/symptoms and regarding the link between behaviour and depression, intention to plan and regularly do healthy activities, beliefs about consequences, derived from the intervention logic model | X | X | X |
| Changes in avoidance and activation over the course of Behavioural Activation for depression | PREMIUM Abbreviated Activation Scale (PAAS) | X | X | X |
| Economic outcomes: Employment status, Household status, Productivity Loss (income and days (hours) of work lost), Out-Of-Pocket Payments (OOP), Opportunity Cost of Time (average wage and time), Borrowing/Selling Assets, Household earnings and expenditure, Catastrophic Health Spending (OOP as % of household expenditure) | Set of questions related to economics outcomes in DiaDeM CRF adapted from Asset index questionnaire | X | X | |
| Health care resource use | Modified client service receipt Inventory (CSRI) | X | X | |
| Medication | Modified client service receipt Inventory (CSRI) | X | X |
* Bonita R, Winkelmann R, Douglas KA, de Courten M. The WHO Stepwise Approach to Surveillance (Steps) of Non-Communicable Disease Risk Factors [Internet]. Global Behavioral Risk Factor Surveillance. 2003. p. 9–22. Available from: http://dx.doi.org/10.1007/978-1-4615-0071-1_3.
At three months post-randomisation, data on the severity of depression, anxiety, health-related quality of life and HbA1c will be assessed only. In addition, data on potential mediators derived from the DiaDeM BA intervention logic model (Figure 1) will be assessed at all time points: knowledge about depression/symptoms; the link between behaviour and depression; intention to plan; regularity of healthy activities; beliefs about consequences of activities. Information will also be gathered to track changes in avoidance and patient activation over the course of BA for depression.51
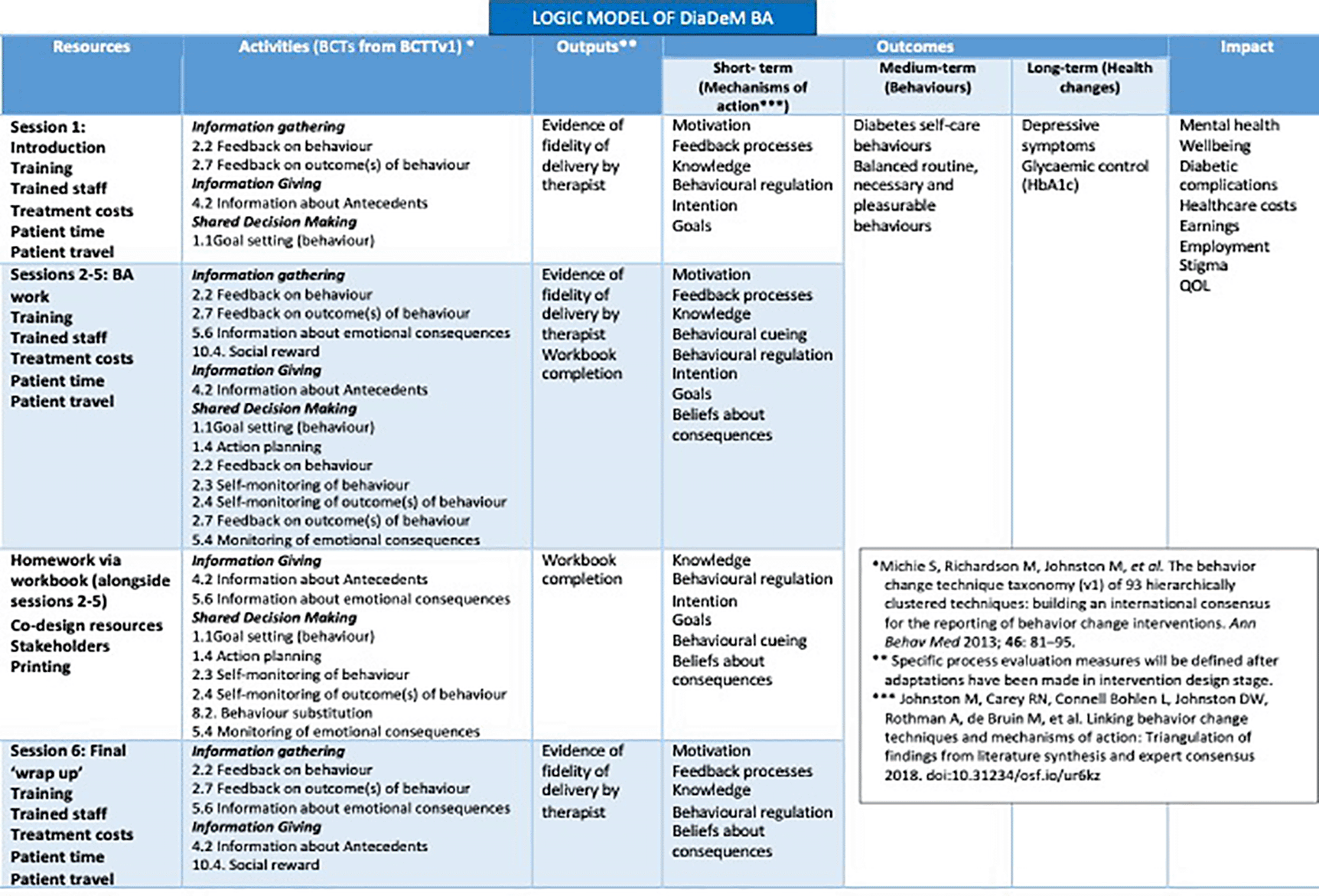
BCT, behaviour change technique; BCTT, behaviour change technique taxonomy; QOL, quality of life.
The baseline and follow-up CRFs data and anthropometric measurements will be entered directly into the remote data collection software Qualtrics (ODK is a free, open source alternative) using tablets at each site. However, for the blood test reports the data will be first entered into a paper format or blood reports log since the reports will include identifiable information. From the log, the RAs will enter the information to the Qualtrics data sheet mentioning only the study ID and the results.
We will collect information on the following feasibility outcomes: 1) recruitment rates, assessed as the number of participants eligible, consenting and randomised, out of those screened; 2) reasons for ineligibility/non-participation/non-consent of participants; 3) length of time required to achieve the required sample size; 4) retention in the study, assessed as the number of participants randomised who are successfully followed up at third and sixth months; 5) retention in treatment reported as the number of sessions attended out of the total number of sessions offered and; 6) intervention fidelity of delivery of the BA intervention. We will also collect information about data completeness, feasibility and acceptability of collecting data using the proposed tools and methods at baseline and at follow-up as a secondary outcome. The schematic diagram of the trial activities is displayed in Figure 2.
A descriptive analysis will be undertaken to describe the baseline characteristics and to report on the feasibility objectives based on the outcomes identified above. Quantitative variables will be presented as mean±SD or as median and interquartile range, depending on their distribution. Absolute and relative frequencies will be used to present categorical variables. Recruitment and completion rates will be estimated and presented alongside 95% confidence intervals (CI). All data will be presented by the trial arm as well as by severity of depression (mild and moderate/severe). All analyses will be conducted in Stata V16 or later (StataCorp, 4905 Lakeway Drive, College Station, Texas, 77845 USA). A CONSORT52 diagram will be provided to display the flow of participants through the study. If a participant withdraws consent to participate, data collected up to the point of withdrawal will be retained and used in the analysis, unless there is a request for withdrawal of all data collected till that point.
A mixed-methods process evaluation will be carried out using the Medical Research Council (MRC) guidance.53 Quantitative data on attendance, drop-out and delivery will be gathered as part of the feasibility trial and will be important in assessing the dose, reach and delivery of the intervention.31,53–55 Sessions will be audio-recorded with written informed consent. To support the quality of reporting and assessment of intervention fidelity, a description or checklist of the core components of each session will create a ‘fidelity index’.53 A number of sessions will be purposively selected (approximately 10% of sessions, a mixture of different sessions) to be listened to and researchers will complete the fidelity index accordingly, this will provide information on the fidelity of the intervention i.e. how closely the intervention was followed as intended.
Semi-structured interviews and/or focus groups will be conducted in each site with approximately N = 12 patients; N = 12 facilitators and N = 8 managers/supervisors immediately following the intervention. The interviews may include techniques such as storytelling, using vignettes/case studies and photo voices to facilitate discussion. The qualitative data will explore experiences of intervention delivery and acceptability and will identify any barriers and drivers to delivery including contextual factors such as the health facility environment or any other factors that may affect delivery and implementation. Unintended consequences of the intervention, mechanisms of change, perceptions on task shifting and staff training needs will also be explored during the different interviews.
We will test methods of collecting service use and other economic data to be used for the economic analysis of the main trial. Health care resource use, medication, out of pocket payments, travel costs, time off work, household assets, productivity loss, opportunity cost of time and wider household expenditure will be collected by patient self-report using the modified Client Service Receipt Inventory (CSRI).56 The EQ-5D-5L will be used to collect information on patients’ health-related quality of life. We will check the appropriateness and completeness of the economic questions.
Participants will be assigned a unique study ID which will be used for all tools and records to ensure anonymity. Confidentiality will be protected by restricting any kind of access to non-authorized persons. All identifiable information of the participants will be stored at each study site securely; electronic records will be kept under password protection and hard copies will be stored in locked filing cabinets. All anonymized data will be transferred to and stored as anonymous data at the University of York, which will act as the data curator. A secure password protected and encrypted electronic database will be set up to store the data. To ensure the quality and completeness of collected data, validation, checking, proofing and cleaning procedures will be carried out by a delegated trial manager at the study sites, followed by a second check by the programme trial data manager according to standard procedures. The data management policy of DiaDeM formulated under the guidance of the University of York trials unit will be followed by the research team. Data will be available upon request to the DiaDeM data governance group following the DiaDeM data-sharing procedures, however, on completion of the trial, the de-identified data will be made accessible through open access repository with clear instructions on how to request for the data. Qualitative interviews will be transcribed in the language of the interview and anonymized but linked with the trial ID before translation for analysis. Digital recordings of the interviews will be stored in a secure, password-protected folder. Once the analysis of the interviews is completed all the recorded versions will be erased from the digital recorders.
The Study Management Group, including the chief investigator, trial leads, site investigators, co-investigators, trial managers, statisticians and program management group members, will supervise the planning and delivery of the project. They will monitor the methods, processes and their implementation by overseeing the trial managers, research fellows and research assistants.
The overall conduct of the trial including the procedures and data will be monitored regularly by an Independent Trial Steering Committee (ITSC), and Data Monitoring Committee (DMC).
The ITSC will comprise a chair, statistician and two members, independent from the sponsor and funder. It will provide independent oversight and supervision to ensure the validity and credibility of the trial, and protocol adherence, in addition to the assessment of the safety of the intervention and protection of the participants. The DMC will comprise three independent members who will safeguard the scientific integrity, validity and integrity of the data, progress and accruing data of the trial and will advise the ITSC on the conduct of the trial in particular the safety of participants. The risks associated with the DiaDeM intervention and the data collection procedures are minimal. Complications and emergencies related to diabetes (like hypoglycaemia, diabetic ketoacidosis, and cardiovascular complications) will be included in the list of potential AEs. Serious adverse events (SAEs) will be defined according to the International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use-Good Clinical Practice (ICH GCP).57 All the related and unexpected AEs will be assessed, recorded and reported to the respective country coordinating centres and concerned personnel, according to the agreed timelines and SOPs. Events will be followed up until the event has been resolved or a final outcome has been reached. The trial may be stopped if guided by ITSC and DMC, as per termination guidelines. At the conclusion of the feasibility trial, ITSC will also advise on the progression to the full trial following the criteria specified in Table 2.
The DiaDeM project received formal ethical approval from the Health Sciences Research Governance Committee (HSRGC), University of York (Ref:HSRGC/2020/409/B), Diabetic Association of Bangladesh (Ref: BADAS-ERC/EC/20/00300), National Bioethics Committee Pakistan (Ref:No.4-87/NBC-578/20/1101), Institutional Research and Ethics Forum of Rawalpindi Medical University (Ref:242/IREF/RMU/2020) and Ethics Committee of Office of Research Innovation & Commercialization (ORIC), Khyber Medical University (KMU), Pakistan (Ref:DIR/KMU/UEC/25). Any amendment to the protocol will be submitted for approval to the same ethics governing bodies.
The study will adhere to the fundamental principles of human rights and dignity laid down in the Declaration of Helsinki.58 Study procedures will comply with legislation and guidance for good practice governing the participation in research of people lacking capacity as set out in the Mental Health Act (UK) 2005.59
The collection, transport and storage of the blood samples of participants will stringently align with the mandates of the Ethics committees, pertinent to confidentiality, transport, storage, ownership and future use of blood samples.
If any abnormality will be detected based on the biochemical assessment or any other screening tests/scales, they will be referred to the concerned specialist, as indicated. If a participant during the trial reveals any suicidal ideation or risk, the facilitators and research assistants will follow the suicide risk protocol specific to each site, including referring to mental health specialist for clinical risk assessment and further management. Unblinding will not be required, since the outcome assessor will be blinded only, whereas the participants, health care providers and recruiting RA will be aware of the allocation group. Any AEs or risks will be reported by the assessor as per SOPs, irrespective of the allocation status of the participant.
Consent
Written Informed consent (via signatures or thumb impressions) for participation in study, anthropometric measurements, collection of blood samples, recording of the intervention sessions, storage and use of the anonymised data will be obtained from all participants. Participants will be informed (via participant information sheet) that their anonymised data may be published in journals and conferences. Those willing to get interviewed for the process evaluation will be provided separate information sheets and hard copies of consent forms to read and record their signatures/thumbprints on the same paper copies.
Dissemination
Community Advisory Panels (CAPs) of the DiaDeM programme, set up at each study site, will meet every 6-months and will advise on and contribute to the dissemination of findings locally. Findings will be disseminated in high impact, open-access peer-reviewed journals, in local and international conferences and as reports for policymakers and stakeholders in the countries involved. Additionally, presenting at national conferences and meetings of professional associations is intended. An open access repository of all materials, syntax, databases and team publications will be created.
The DiaDeM feasibility trial has been launched on 29th March 2022 and the recruitment has been completed for 128 participants on 12th May 2022. The intervention sessions have been commenced and the participants will be followed up at 3-month and 6-month post-randomization. The trial was registered on 14/04/2022 (ISRCTN 75501608).
Healthcare systems face a key challenge of mental-physical multimorbidity.60,61 People with chronic physical health conditions, such as diabetes have approximately 2 to 3 times higher risk of mental illness, most commonly depression.5,6 While diabetes is amongst the four most common NCDs worldwide,62,63 the prevalence of diabetes is following an acute trajectory in South Asian countries64 with rates of depression in Pakistan being 16.7%3 and in Bangladesh being 12.5%.4 Furthermore, diabetes is amongst the top five causes of years lived with disability.65 Diabetes and depression often co-occur,6 adversely affecting each other’s outcomes.66 Estimates of depression and diabetes range from 13% to 18% with mild depression often being undiagnosed.67 Depression with diabetes is associated with poorer self-management and treatment adherence/response, poorer glycaemic control and diabetes complications, longer duration and recurrence of depressive episodes, increased healthcare costs, poorer quality of life and higher morbidity and mortality.68
Systematic reviews have supported the effectiveness of psychological interventions for depression and glycaemic control in people with diabetes.69 However, the effect of these interventions on diabetes-related complications and mortality has not been examined. None of the included studies was undertaken in LMICs. BA has been reported to be as effective as CBT in the treatment of depression yet BA is less resource-intensive than CBT. A systematic review reported BA was effective for depression compared to usual care in selected long-term conditions,70 however, there were no studies for BA in depression-diabetes globally and in LMICs specifically.
By integrating the management of depression in diabetes care, there is potential to improve mental health, quality of life and diabetes complications. Our candidate intervention, ‘Behavioural Activation’, is a relatively simple, flexible and effective talking therapy for depression.20 It has also been adapted in community pharmacies mood intervention study (CHEMIST) to be delivered by non-specialists and for multimorbidity in the UK. We propose to integrate the delivery of BA with diabetes care, tailoring it to promote diabetes self-management activities and improve mood.
Both Pakistan and Bangladesh, have diabetes specialist services which are relatively well developed. However, mental health services are not provided in these specialised care centres. Integrating recognition and treatment of depression within diabetes care offers an opportunity to address the large mental health treatment gap71 and also help improve health and economic outcomes.72
Open Science Framework: Extended Data of DiaDeM Feasibility Trial Protocol. https://doi.org/10.17605/OSF.IO/DGQ74.73
This project contains the following extended data:
Open Science Framework: SPIRIT and TIDieR checklists for ‘An adapted behavioural activation intervention (DiaDeM) for people with diabetes and depression in South Asia: A feasibility study protocol’. https://doi.org/10.17605/OSF.IO/DGQ74.73
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors wish to express their appreciation to all the members of the community advisory panels in Pakistan and Bangladesh, for their review and feedback on the planned trial, its methods, feasibility and suggestions on the adaptation of the DiaDeM intervention during the co-design workshops. They are also profoundly grateful to the clinical staff and the head of departments of all the study sites in Pakistan and Bangladesh who had been instrumental in the site preparations and planning for the conduct of the trial apart from their invaluable feedback during codesigning for the adaptation of DiaDeM.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)