Keywords
nicotine patch, pharmacokinetics, never-smokers, overdose, mood
nicotine patch, pharmacokinetics, never-smokers, overdose, mood
A growing number of studies are administering nicotine to never-established smokers in attempts to assess the effects of nicotine that are independent of withdrawal alleviation (e.g., Duke et al., 2015; Hahn et al., 2020; reviewed by Heishman et al., 2010; Knott et al., 1999). However, there has been surprisingly little formal assessment of dose and pharmacokinetic factors that can result in aversive responses to nicotine. This potential for overdose is especially true in the case of non-smokers. It has long been known that excessive and rapidly administered acute doses can cause nicotine overdose events (NODEs), including malaise, nausea, sickness, dizziness, unpleasantness, negative affect, and vomiting (Nyberg et al., 1982) and that high and rapidly administered acute nicotine doses can result in NODEs even in nicotine-dependent individuals (Gilbert et al., 1992; Seyler et al., 1984, 1986). Very little attention has been given to the potentially confounding effects of NODEs in never-established smokers (for brevity never-smokers are those smoking fewer than 100 cigarettes in their life) administered nicotine by nicotine patch, gums, and other routes. Given that NODEs are likely to decrease positive affect (PA), increase negative affect, and divert attention away from appropriate task performance in studies assessing the effects of nicotine on attention and affect, it is critical that studies use means of nicotine administration that minimize NODEs if inferences concerning the inherent effects of self-administered nicotine are to be accurately characterized and to have ecological validity. The accurate characterization of the effects of acute nicotine administration on mood and other potentially rewarding effects is important because never-smokers are the only group that can progress to becoming smokers and it is important to understand motives for progression to smoker status. Nicotine enhances dopamine in brain reward-related regions, such as the nucleus accumbens, with PA-inducing effects that have been promoted by many as critical determinants of the development-dependent smoking (Volkow et al., 2019).
There are several reasons why the better characterization of different nicotine dosing products and regimens is critically important to understanding nicotine use and treatment of nicotine dependence via smoking and other means. For example, while high doses of blood nicotine resulting from the rapid smoking of two 2.4 mg nicotine cigarettes after overnight deprivation in habitual smokers lead to large increases in blood beta-endorphin (BE) concentrations, minimal or no increases have been observed after the smoking of normal-nicotine delivery cigarettes smoked at more normal rates (Gilbert et al., 1992, 1994). Of note, BE, like cortisol and adrenocorticotropic hormone (ACTH), have been found to correlate strongly with the degree of aversive symptoms (e.g., nausea, sickness, and unpleasantness) in such studies (Gilbert et al., 1992; Seyler et al., 1984, 1986) associated with the smoking of high nicotine-delivery (e.g., 2.4 mg) cigarettes. Self-reported NODEs (e.g., nausea, sickness, and unpleasantness) increased after smoking two 2.4 mg nicotine cigarettes in rapid succession and subjective distress was correlated with changes in blood BE and cortisol. However, more natural smoking of a single regular (1.0 mg) cigarette did not increase blood BE or cortisol or subjective distress in a study by Gilbert et al. (1992). Overall, these findings indicate the critical importance of dose in determining the effects of nicotine in smokers, but there is no characterization of the effects of these dosing parameters in never-smokers and non-smokers.
Nonetheless, because of the lack of formal assessment of NODEs in the previous studies in never-smokers, the present study assessed NODEs formally by questionnaire every 20 minutes beginning 20 minutes after patch application in an investigation designed to assess the effects of nicotine on mood and attentional processes in never-smokers. The present report first describes the prevalence and severity of NODEs during the two-hour period after patch administration for two different brands of 7 mg nicotine patches, one with slow-rise and one with a fast-rise blood nicotine concentration, with nominally the same 24-hour nicotine delivery but with different pharmacokinetic properties.
This study was approved by the Southern Illinois University Carbondale (SIUC) Institutional Review Board, IRB protocol number: 99128, assurance number FWA00005334. The study was initially approved on January 25th, 2004, and data collection started shortly after approval on April 30th, 2004. The study ran until February 1st, 2006, and the IRB re-reviewed and approved the protocol on January 18th, 2006, as part of a standard procedure of updating active studies (i.e., no ethical breaches occurred). Participants consented to participate at the beginning of the study and were able to quit the study at any point. NODE symptoms resolved within 30 minutes of patch removal. Unlike previous studies that lacked a formal assessment of NODEs in never-smokers, we assessed NODEs every 20 minutes beginning 20 minutes after patch application in an investigation designed to assess the effects of nicotine on mood and attentional processes in never-smokers. The present report first describes the prevalence and severity of NODEs during the two-hour period after patch administration for two different brands of 7 mg nicotine patches, one with fast-rise (study phase 1) and one with a 7 mg slow-rise blood nicotine concentration (phase 2), with nominally the same 24-hour nicotine delivery but with different pharmacokinetic properties. The primary aim of the study initially was to assess the effects of acute nicotine on PA in never-smokers, but due to the unexpected modestly high prevalence of NODEs observed in our initial group of participants, our subsequent aims were extended to include the comparison of the subjective states of fast versus slow blood nicotine rise-time patches in never-smokers.
Participants were never-smokers (n = 67) between the ages of 18 and 47 years (Mage = 23.76; SD = 7.16; 50% female). The sample size was dependent on funding. Never-smokers were defined as those smoking fewer than 100 cigarettes in their life and none in last year (Klemperer et al., 2021). The age limits were used because of the legal age of smoking (18 years old) and potential health risks and limited relevance to smoking onset at older ages. Participants were recruited by adverts throughout the Midwestern University community. Phone and in-person screening interviews were used to assess inclusion and exclusion criteria. Exclusion criteria included reported use of psychoactive drugs or medications other than caffeine, marijuana, and alcohol, excessive alcohol use, aged less than 18 or more than 50 years, non-English speaking, atypical sleep cycles, and serious medical problems. Participants were instructed not to drink alcohol for the 12 hours preceding each of the experimental sessions and not to smoke or use marijuana or any other psychoactive substance for at least 72 hours prior to the session. Only those who adhered to these abstinence requirements were included in the data analysis. Some participants were removed because they reported significantly high nicotine overdose symptoms 20 min and 40 min post-patch application (n = 6), prior to the time of significant increases in blood nicotine concentration and therefore presumably due to minor illness or other non-nicotine causes. No participants experienced NODEs severe enough to withdraw their data from the study. A total of 27 participants received the fast-rise patch and 40 received the slow-rise patch.
Feelings State Questionnaire (FSQ). Participants completed the FSQ developed by the author (Gilbert et al., 1992), a measure designed to assess the physical and affective effects of nicotine. The FSQ contains 14 10-point Likert items ranging from 1 = none to 10 = extreme. We used summated scores for the analyses. We used two of its subscales, 1) nicotine overdose symptoms (sick, dizzy, lightheaded, and nauseous) and 2) positive (happy and pleasantness). Previous research has shown that the FSQ is sensitive to experimental manipulations and correlates with measures of mood (Gilbert & Spielberger, 1987). The internal consistency of the four-item overdose scale at time one for the placebo session was acceptable, α = .680 and excellent for the PA scale, α = .914. The mean score on the nicotine overdose scale indicated low overall overdose symptoms (M = 2.07, SD = 2.43) and was normally distributed, γ = 1.10, κ = -.10. The mean score on the PA scale indicated moderate overall PA (M = 10.60, SD = 3.59) and was normally distributed, γ = -.34, κ = -.31.
During phase 1 of the study, a 7 mg NicoDerm® CQ® rapid blood-nicotine rise patch was used because the series of NicoDerm® CQ® 21 mg, 14 mg, and 7 mg patches are one of the two most widely used brand of patch for smoking cessation in the USA (personal search of ClinicalTrials.gov) and because a number of previous studies by others had used either the 7 mg, 14 mg, or 21 mg NicoDerm® CQ® patch without reporting or apparently formally assessing NODEs (Barr et al., 2008; Potter & Newhouse, 2008; Wignall & de Wit, 2011). GlaxoSmithKline provided the patches. According to the findings of Gupta et al. (1995) NicoDerm® CQ® patches (i.e., fast-rise) reach 80% maximum blood nicotine concentrations about two hours after application, while the formulation of the Habitrol® patches (i.e., slow-rise) used at the time of the study reached 80% maximum blood nicotine concentrations about four hours after application. From one to two hours after application the NicoDerm® CQ® patch results in a blood nicotine concentration that is twice or more than that of the Habitrol® patch. It is important to note that the blood nicotine rise times for the “rapid-rise” patch patches are relatively faster than other patches and are still much slower than for other forms of over-the-counter nicotine administration, including lozenges, inhalers, e-cigarettes, and tobacco cigarettes (Shiffman et al., 2005).
A MiniCO meter (Vitalograph, Lenexa, Kansas) was used to assess carbon monoxide concentrations prior to acceptance into the study and at the beginning of each of the two experimental sessions in order to help assure never-smoker status and abstinence from other smoked psychoactive substances. We used a cut-off of less than or equal to six parts per million.
Participants completed an orientation session during which they were informed of the nature of the study, signed an informed consent approved by the Carbondale Committee for Research on Human Subjects (protocol #99128), and completed a current and life-time retrospective version of the Time-line Followback for Tobacco, Drugs, and Substance Use (not used in the current study; Sobell & Sobell, 2000), the FSQ (Gilbert et al., 1992), a demographics questionnaire that assessed age and gender, and provided a carbon monoxide breath samples. Participants attended two experimental sessions during which they sat alone in a small experimental room that was electronically connected to a central control room. The control room contained a server computer for control of experimental tasks, video display units for monitoring each participant’s computer and behavior, and audio communications with each of the individual subject rooms. Participants earned monetary compensation for the completion of the study. Half of the never-smokers wore a nicotine patch on the first session and a placebo patch on the second session, while the other half had the reverse order of patches. The participants and the experimenters interacting with the participants were blind to the nicotine versus placebo status of the patches.
Experimental sessions began between noon and 2:00 p.m. and lasted about 120 minutes. A minimum of one day and a maximum of seven days separated the practice sessions and each of the four experimental sessions. The nicotine or placebo patch was then applied. A researcher not involved in data collection or otherwise interacting with subjects provided the appropriate nicotine or placebo patch and kept a record of which patch was given. Neither the participant nor the researchers who gathered the data and interacted with the participant had any information about which patch was given.
During phase 1 of the study (n = 27 participants) a 7 mg NicoDerm-CQ® (rapid blood-nicotine rise) patch was used because this is the most widely used brand of patch for smoking cessation and patch research in the USA. After the identification of a modest number of incidences of adverse reactions to the NicoDerm-CQ® patch, we switched to a slower blood nicotine rise patch in what we now label as phase 2 of the study that used all new participants (n = 40). To avoid nausea and other adverse effects of rapidly rising nicotine, we used a 7 mg Habitrol® patch that has a slower blood nicotine rise profile than the NicoDerm-CQ® patch. The active patches were purchased at a local pharmacy. The placebo patch was a 2” × 2” bandage. To minimize the ability of participants to differentiate between active and placebo patches by skin sensations (itching or irritation) we used a cover bandage with capsaicin cream. Both the active and placebo patches were placed in the center of a 6.5 cm × 7.0 cm cover bandage. Then, a small amount (.05 cc) of capsaicin .075% cream (Capzasin-HP7, Chattem, Inc) was applied to the Teflon-coated surface of the cover bandage, covering an area 5 mm wide immediately next to each of the edges of the bandage. Pilot testing demonstrated that this procedure eliminated the ability of subjects to detect differences between the active and placebo patch when applied. During the time after patch application, participants completed the FSQ starting 20 minutes after patch administration and every 20 minutes after for 100 minutes.
We analyzed the data using SPSS Statistics 27.0 (RRID:SCR_016479) (IBM Corporation, LLC, Armonk, NY, 2020). We conducted a 2 (patch type: slow-rise vs. fast-rise) × 2 (condition: placebo vs. nicotine) × 5 (time: 20 minutes vs. 40 minutes vs. 60 minutes vs. 80 minutes vs. 100 minutes) analysis of variance (ANOVA). We conducted one ANOVA for the overdose scale and one ANOVA for the PA scale using the Greenhouse-Geisser degrees of freedom correction to adjust for the false inflation of the effect size due to heterogeneity of variances. A Bonferroni correction for all post-hoc tests to control for family-wise error was used.
All 67 participants completed all five timepoints. There was a three-way interaction, F(2.14, 89.78) = 10.24, p < .001, = .196, involving patch type, condition, and time (Stone, 2022). There were significant simple effects for the fast-rise patches between conditions at 60, 80, and 100 minutes after patch administrations, ps < .001 (see Figure 1). At these times after patch administration, individuals who used the fast-rise nicotine patch reported significantly more NODEs compared to when they received the placebo patch. For the PA scale, there was a three-way interaction, F(2.84, 119.31) = 3.19, p = .028, = .071, such that there were significant simple effects for the fast-rise patches between conditions at 60, 80, and 100 minutes after patch administrations, ps < .016 (see Figure 2), such that individuals who used the fast-rise nicotine patch reported significantly less PA compared to when they received the placebo patch. There were no significant differences between the placebo patch and the slow-rise nicotine patch at any time points for either scale, ps > .396.
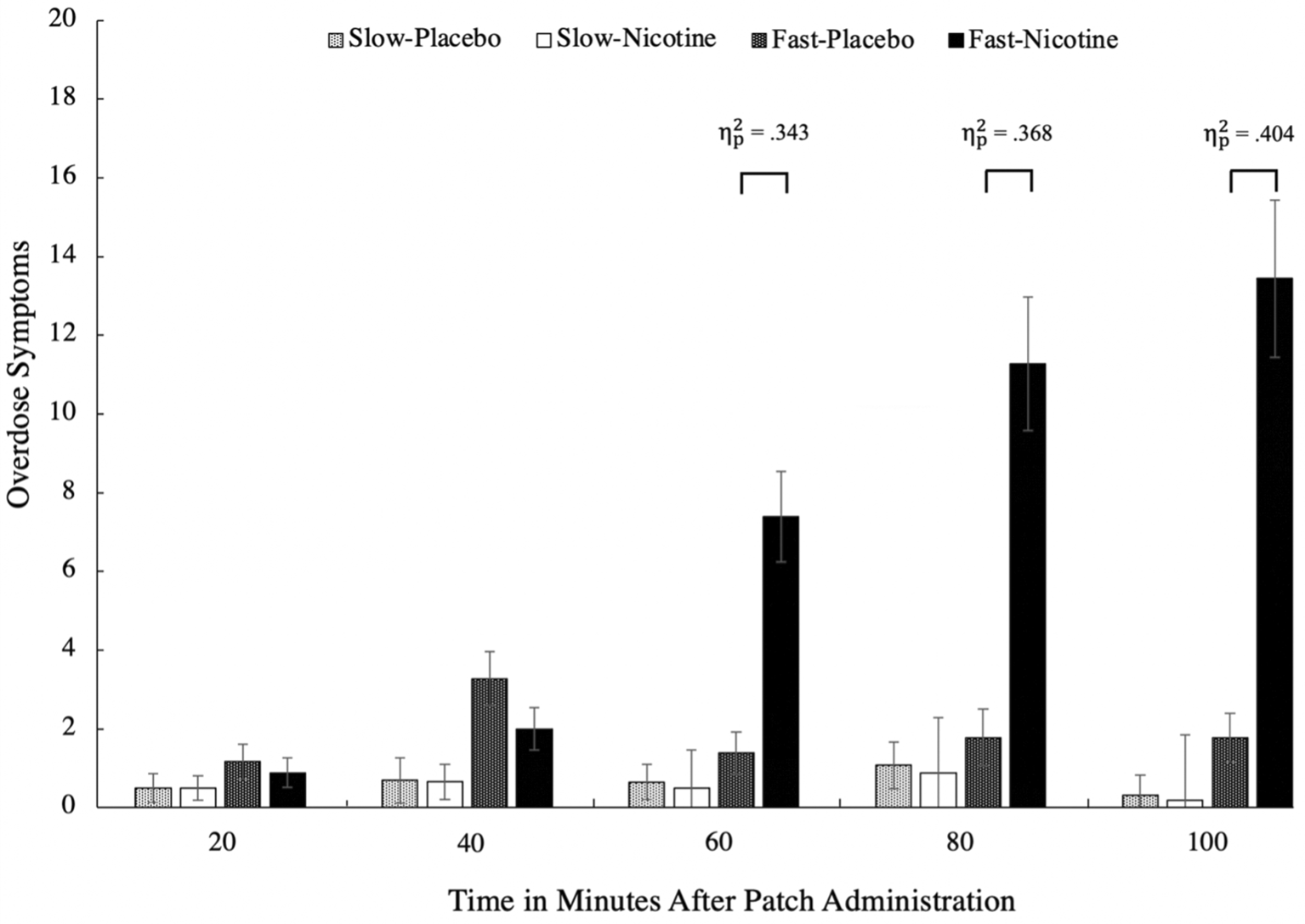
Mean NODE scores between the two patch types (slow-rise vs. fast-rise) by condition (nicotine vs. placebo patch) across the five time points in 20-minute intervals in a three-way ANOVA. The results show a large effect of NODEs between the fast-rise compared to the placebo conditions, where only the fast-rise patches caused significant NODEs. NODE, nicotine overdose event.
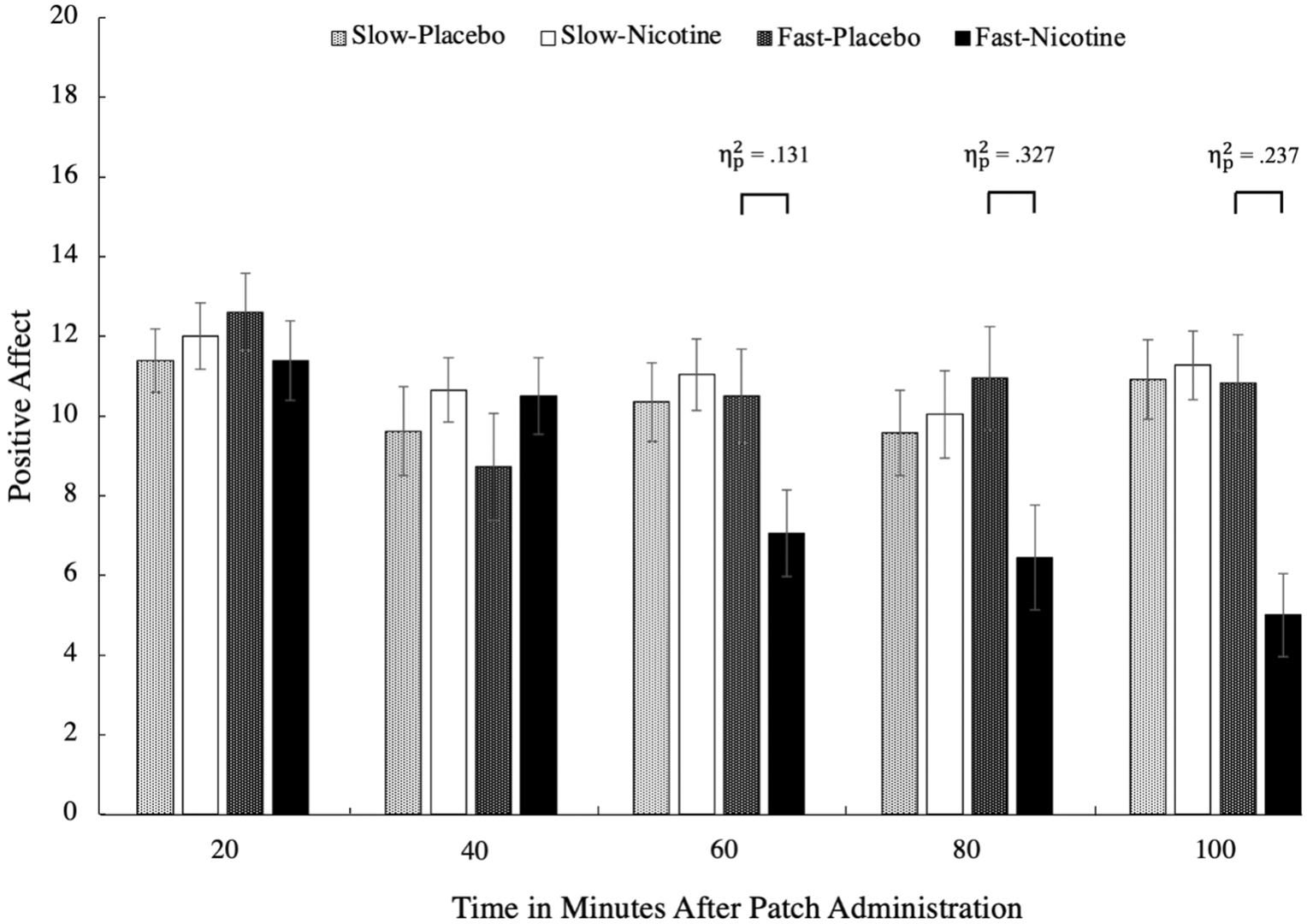
Mean PA scores between the two patch types (slow-rise vs. fast-rise) by condition (nicotine vs. placebo patch) across the five time points in 20-minute intervals in a three-way ANOVA. The results show a large effect of PA between the fast-rise compared to the placebo conditions, where only the fast-rise patches caused a significant reduction in PA. PA, positive affect.
We assessed the prevalence of NODEs by comparing baseline scores to that of T5. We used a mean score change of 2 points to determine which participants experienced NODEs or a reduction in PA. The findings suggest that in never-smokers, the lowest dose (7 mg) of rapid blood nicotine-rise patches are associated with a high prevalence of NODEs (45.83%) and decreased PA (54.17%) compared to a placebo patch (8.34% for NODEs and 33.34% for PA). Note that none of the items were significantly more endorsed than the others (i.e., these changes in NODEs and PA were not driven by a single item).
The current investigation is to compare the subjective effects of two different commonly used nicotine patches in never-established-smokers. We found that never-smokers reported significantly more NODEs and less PA compared to placebo. These effects were not found in never-smokers who received the slow-rise nicotine patch during our two-hour assessment period. Never-smokers in the present study experienced adverse NODEs, such as nausea and feeling of sickness, when placed on the nicotine patch that produces a rapid increase in blood-nicotine concentration. Below we briefly address the potential implications of these findings, the limitations of the current study, and directions for future research.
The present findings are consistent with previous studies on habitual smokers that have shown that forcing regular smokers to consume nicotine more quickly than their typical rate can lead to nausea and even vomiting (e.g., Gilbert et al., 1992) smoke more intensely. For example, administering nicotine too quickly or in too high of doses can produce NODEs. There is a fine line between beneficial and distress-producing doses (Gilbert et al., 1992). Small differences in nicotine dosing (e.g., doubling the dose) can produce large differences in subjective and physiological effects (Gilbert et al., 1992) that make NODE-related findings ecologically invalid. Administering nicotine too quickly or in too high of doses can produce NODEs. Because habitual smokers typically do not experience NODEs when using nicotine, it is suggested that the doses of nicotine that produce NODEs may not be ecologically valid in many cases. The present results call into question the results of studies using fast-rise nicotine patches in non-smokers (e.g., Griesar et al., 2002; Sørensen et al., 2009). NODEs in nicotine research may interfere with the detection of potential reinforcing, affect-modulating, and attention-enhancing effects of nicotine and be correlated with abnormal neural and hormonal activity, limiting ecological validity.
For practical purposes, most laboratory studies of acute dosing effects in never-smokers use experimental sessions that last no more than half a day each, a period that included nicotine administration followed by mood and experimental tasks. In addition, blood nicotine level figures from the pharmacogenetics study (Gupta et al., 1995) show that fast-rise patches produce elevations at two hours that are twice as high as the slow-rise patches. However, eight hours after application the two patches produce equally high blood nicotine concentrations. The time trajectories of NODEs across this period are not known. Given that rapid tolerance develops to acute nicotine, it is not clear whether the slow-rise patches would ever produce a substantial prevalence of NODEs or for how long a duration NODEs would be sustained in rapid-rise participants. It would likely be fruitful to examine NODE time courses and to evaluate self-paced and other means of nicotine administration (e.g., electronic cigarettes or inhalers) in never-smokers. We believe that it is critically important for future acute administration studies to systematically assess NODEs using our measure or similar measure to help ensure that they are not adversely affecting study outcomes and conclusions.
The findings have a few limitations and future directions. First, we stopped data collection for the never-smokers who received the fast-rise nicotine patches because the patches were making the participants significantly sick (e.g., several participants felt they might vomit), something that we feared would prevent the accurate assessment of the effects of nicotine on PA. Thus, individuals were not randomized to the two patch types. However, it is important to note that the subjects were from the same larger subject pool (all recruitment procedures and acceptance criteria remained the same for the two samples), there was no recruitment break between the samples, and the two groups did not differ in terms of demographics.
Further, despite the dates of data collection occurring in the early 2000s, the results should not be affected by changing cultural factors since the data were collected. The NicoDerm and Habitrol patches are still being used in studies and sold over the counter. Thus, our findings are still relevant and may be even more relevant as researchers continue to use fast-rise patches with never-established smokers, despite the loss of ecological validity from the excessive presence of NODEs in their participants.
In conclusion, our findings make an important contribution to the literature in that they underline the oft-neglected importance of carefully and routinely characterizing NODEs because even subtle differences in dosing methods can lead to adverse effects on mood state. The first author noted this some 30 years ago in the case of experimental demands for high levels of smoking-delivered nicotine intake that result in NODEs in dependent smokers (Gilbert et al., 1992). Whatever the assessed subject group, it is critical that researchers to avoid using experimental demands that result in rapidly rising or high blood concentrations of nicotine that cause NODEs and altered cognitive and affective states.
Open Science Framework: Overdose Symptoms and Positive Affect in Never-Established Smokers are Moderated by Nicotine Patch Type. https://doi.org/10.17605/OSF.IO/VS7JC (Stone, 2022).
This project contains the following underlying data:
- Data.sav (spreadsheet data)
- Labels.docx (codebook for spreadsheet data)
- Feelings State Questionnaire.docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Andrews P, Vega JN, Szymkowicz SM, Newhouse P, et al.: Effects of open-label transdermal nicotine antidepressant augmentation on affective symptoms and executive function in late-life depression.J Affect Disord. 2024; 362: 416-424 PubMed Abstract | Publisher Full TextCompeting Interests: I undertake contract scientific work for tobacco and nicotine product manufacturers; I am a Scientific Advisory Board member with Qnovia Inc, a developer of an inhaled nicotine smoking cessation therapy; I am a non-executive director with Advanced Inhalation Rituals, a manufacturer of tobacco products.
Reviewer Expertise: Nicotine pharmacology; biomarkers of exposure and of biological effect; behavioural studies of nicotine use.
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: oncology and lung cancer
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Smoking Vaping (former chaest physician)
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 16 Aug 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)