Keywords
Porphyria cutanea tarda, hemochromatosis, hydroxychloroquine
Porphyria cutanea tarda, hemochromatosis, hydroxychloroquine
Porphyria cutanea tarda (PCT) is the most common type of porphyria and results from a decline of uroporphyrinogen decarboxylase (UROD), an enzyme involved in heme synthesis and resulting in accumulation of uroporphyrin.1 There are two types of PCT. Type 1 is an acquired disorder triggered by iron overload or viral infection. Type II is an autosomal-dominant genodermatosis.2 The cutaneous manifestations arise when cutaneous porphyrins absorb ultraviolet radiation (UV) and generate reactive oxygen species (ROS) that induce skin photosensitivity, sub-epidermal blistering, erosions, milia and scar formation.3 We present a case of a patient diagnosed with type 1 PCT and hemochromatosis, successfully treated with low dose hydroxychloroquine.
A 67-year-old Caucasian male, who worked as a plumber, presented with a two-month history of skin fragility and pruritic blisters on dorsal hands, spreading up the arms. He noted that broken blisters healed with firm little “white spots” and described lethargy and darkening of urine. For many years he had regularly consumed three cans of beer daily. Examination revealed intact blisters alongside numerous erosions over the hands and elbows and milia on inspection of the left hand (Figure 1). There was no forehead hypertrichosis.
The clinical differential diagnosis included hepatocutaneous porphyria and epidermolysis bullosa acquisita (EBA). Skin biopsy, blood, urinary and faecal porphyrins were performed and he was advised to reduce alcohol consumption and sun exposure.
Histopathology revealed an intact sub-epidermal blister with minimal dermal inflammation (Figure 2). Haematological investigations demonstrated; C282Y hemochromatosis (HFE) gene mutation, mildly elevated liver function tests (LFTs) consistent with alcohol intake and elevated ferritin (756 μg/L). Hepatitis and HIV serology were negative. The porphyrin screen showed elevated urinary total porphyrin (3.4 μmol/L), uroporphyrin (3.2 μmol/L), faecal total porphyrin (330 μmol/L), Isocoprophyrin (125 μmol/L), plasma porphyrin (207 nmol/L) and red cell porphyrin (1.8 μmol/L rbc). The patient was referred for venesection but had a major syncopal episode and declined further venesection. Treatment was then commenced with oral hydroxychloroquine 5 mg daily (compounded extemporaneously) and up-titrated over six months to 200 mg daily. Within 12 months the PCT was in complete remission with normal LFTs and ferritin (462 μg/L) and hydroxychloroquine was ceased. The porphyrin screen showed normal levels of urinary total porphyrin (0.17 μmol/L), red cell porphyrin (0.41 μmol/L red cells) and plasma total porphyrin (13 nmol/L).
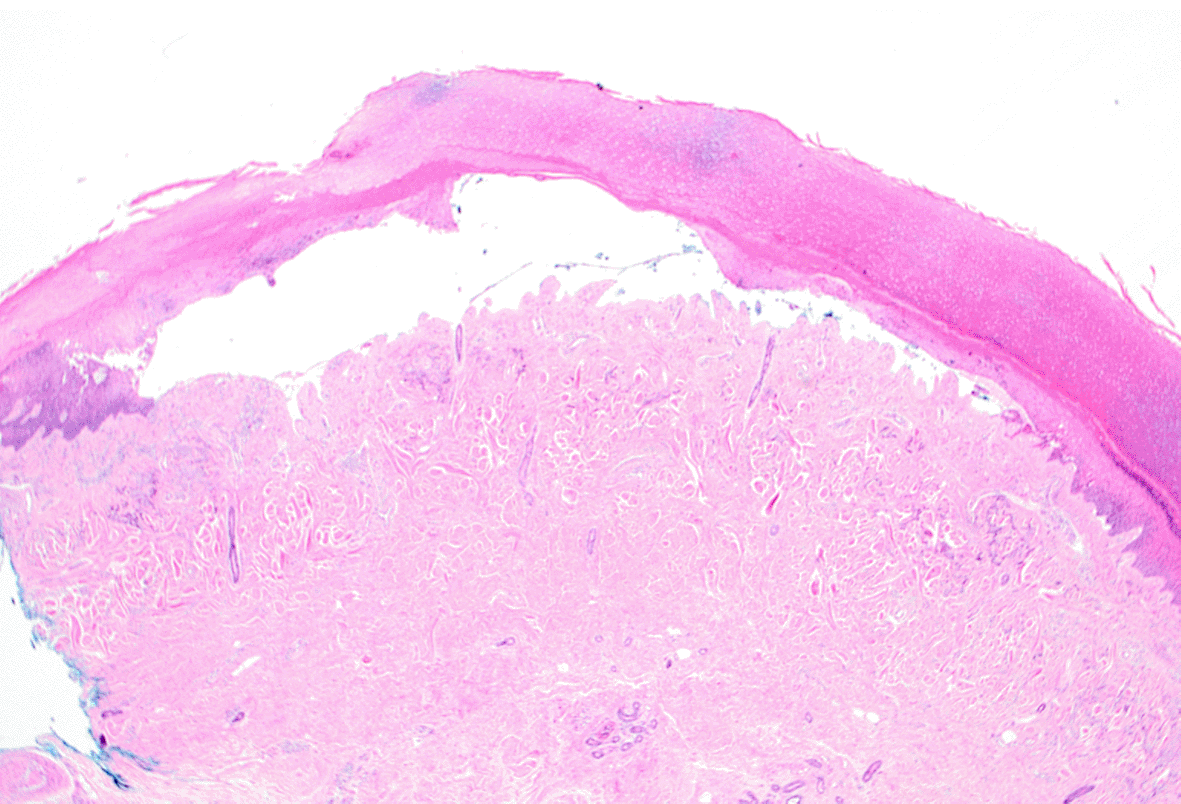
In the superficial dermis there are some thick-walled blood vessels, which stain for Periodic acid–Schiff (PAS). In the upper dermis there is a sparse lymphocytic infiltrate.
The most common triggers for type 1 PCT are alcohol, environmental chemicals, hemochromatosis and viruses.2 Our patient possessed two of the above triggering factors (alcohol and hemochromatosis). Alcohol may trigger PCT by increasing iron absorption through dissociating iron from its binding proteins as well as directly inhibiting UROD.4 Hemochromatosis causes iron excess and can trigger PCT by catalysing ROS formation and increasing uroporphyrin concentration through direct oxidation of uroporphyrinogen and inhibition of UROD.1
Hydroxychloroquine increases porphyrin excretion in PCT and is as effective as phlebotomy with remission in 6–9 months.5 The HFE mutation type appears to be important in hydroxychloroquine therapeutic response, with homozygosity for the C282Y mutation resulting in treatment failure, but heterozygosity, as demonstrated in our case, did not.6 The use of compounded low-dose hydroxychloroquine minimised risk of acute toxicity and provided a safe and effective alternative to venesection in our patient. The dose was up-titrated until clinical remission was achieved. A limitation of this case report was the small sample size of only one patient.
Oral hydroxychloroquine provided a safe and effective alternative to venesection in this patient who was needle phobic.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Rare liver diseases, iron overload disorders (hemochromatosis, ferroportin disease, hemoglobinopathies), porphyrias
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Liver diseases. Porphyria.
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Kowdley KV, Brown KE, Ahn J, Sundaram V: ACG Clinical Guideline: Hereditary Hemochromatosis.Am J Gastroenterol. 2019; 114 (8): 1202-1218 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Porphyria
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 17 Aug 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)