Keywords
Hughes-Stovin Syndrome, Pulmonary artery aneurysms, Deep venous thrombosis, Behçet disease
Hughes-Stovin Syndrome, Pulmonary artery aneurysms, Deep venous thrombosis, Behçet disease
Hughes-Stovin syndrome (HSS) is a rare disorder with high morbidity and mortality rates that was first identified in 1959. It is characterized by the coexistence of deep in situ vascular thrombosis and pulmonary artery vasculitis. HSS is also associated with Behçet’s disease (BD). The main symptoms of HSS include recurrent episodes of deep venous thrombosis (DVT), typically affecting the lower extremities, and the development of PAA, which can lead to life-threatening complications such as pulmonary hemorrhage or aneurysm rupture.1–3
The occurrence rate of Hughes-Stovin syndrome is extremely low, with only a limited number of cases reported in the medical literature.2 It primarily affects young adults, predominantly males. The exact cause of HSS remains unknown, and various etiologies have been proposed. Interesting hypotheses suggested that Hughes-Stovin syndrome may be attributed to an autoimmune disorder, whereas others propose a genetic predisposition or a potential combination of both factors.4,5
The management of Hughes-Stovin syndrome is controversial due to its rarity and limited understanding. The primary objective of treatment is to prevent or control the complications associated with DVT and PAA. Anticoagulation therapy based on vitamin K antagonists is commonly used to prevent further DVT formation. Corticosteroids and immunosuppressive drugs like cyclophosphamide are also prescribed to reduce inflammation and prevent the development and progression of PAA as they are associated with a better outcome.2
These therapies are not based on robust scientific evidence due to the lack of large-scale clinical trials and the limited number of reported cases. The rarity of the syndrome makes it challenging to conduct robust research studies to evaluate different treatment strategies. Furthermore, the optimal duration of anticoagulation therapy and the appropriate dosages of immunosuppressive agents are still areas of debate.2
We report here five cases of HSS associated with BD (Table 1). All of our patients have been hospitalized in Fattouma Bourguiba University Hospital. An interesting variation of the nature and the severity of the symptoms is reported. This is linked to the primary lesions associated with each case. High doses of corticosteroids, immunosuppressants and anticoagulation are the three pillars of the treatment for each patient. We proposed embolization and embolectomy for the most extreme case.
We also included a systematic literature review of the reported cases in the literature and a brief discussion section presenting the different hypothesis for the pathophysiology of HSS.
A 37-year-old North-African unemployed man with a family history of BD presented to the emergency department with a first episode of mild hemoptysis. Physical examination revealed bipolar aphthosis and pseudo folliculitis.
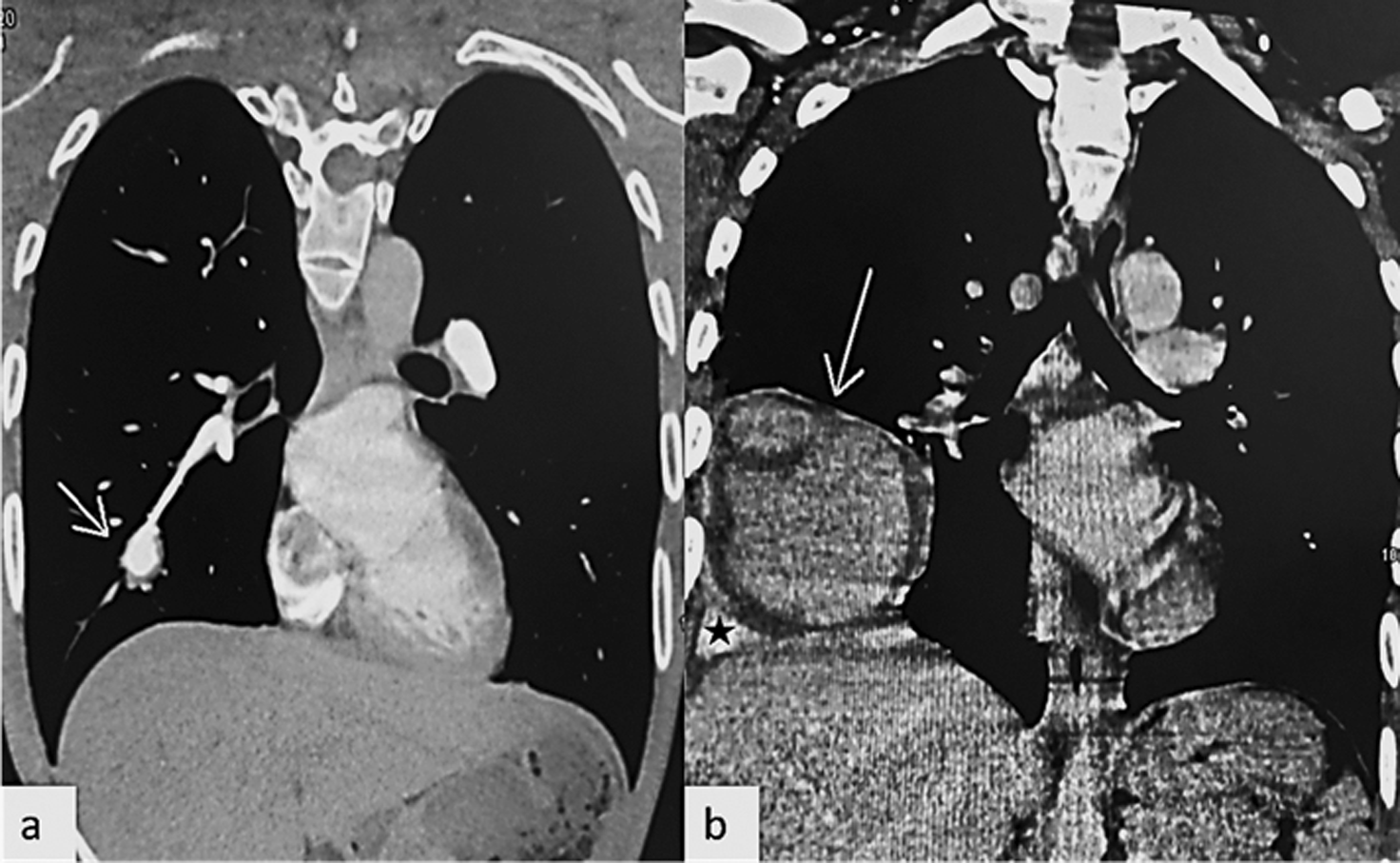
Several artery aneurysms were detected by computed tomography (CT) angiography in both lungs, partially thrombosed, with the largest aneurysm measuring 24 mm (Figure 1a) involving the right lower lobe antero-basal segmental pulmonary artery with in situ mural thrombus. The main challenges were to rule-out the differential diagnosis and to start the IV treatment as soon as possible.
Given the association with BD and after exclusion of other differential diagnoses such as large-vessel arteritis, Wegene’s granulomatosis, systemic lupus erythematosus and connective tissue diseases, we highly suspected the diagnosis of HSS after clinical examination and pulmonary angioscan in the first day of presentation. We concluded to the diagnosis of HSS after receiving lab results ruling-out differential diagnosis. The patient was admitted to the Internal medicine department and treated by three pulses a day of methylprednisolone (1g) since the first day of presentation. These pulses were relayed by oral prednisone (1mg/kg/day). Colchicine (1mg/day per os) was also given as soon as the diagnosis was established.
The patient was readmitted six months later following another episode of hemoptysis accompanied by chest discomfort. His coagulation tests were within normal ranges, and his vital signs were normal. Both urine and sputum microbiological samples were negative for tuberculosis.
Chest CT angiography revealed a marked increase in the size of the right lower lobe antero-basal segmental artery aneurysm from 24 mmm to 116 mm in diameter, with intra-aneurysmal adherent in situ thrombosis and ipsilateral alveolar and pleural bleeding due to extra-luminal acute leak (Figure 1b). Chest CT showed also endoluminal defect in the posterior and latero-basal branches of the right pulmonary artery, related to acute pulmonary embolism. No additional testing or diagnostic challenges were noted as the patient was already being managed for this pathology. Treatment consisted of blood transfusion, antibiotics, and methylprednisolone 1g/day for three days, then cyclophosphamide (2000 mg/m2, IV). After stabilizing his vital signs, he had an embolization of the right lower antero-basal segmental pulmonary artery aneurysm, see Figure 2.
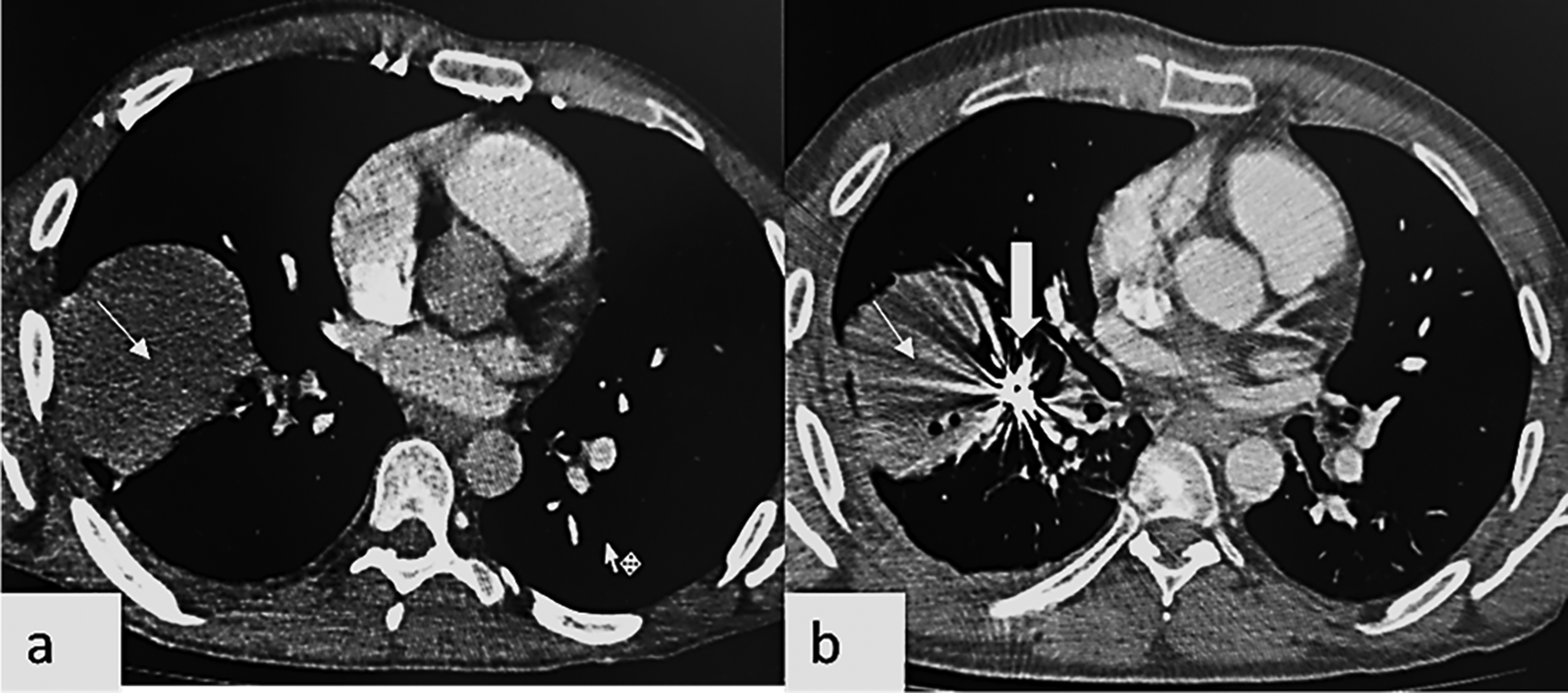
A follow up chest CT angiography one year later showed multiple new pulmonary arteries aneurysms affecting the right upper lobe ventral segmental artery (Figure 3a) and the right lower lobe apical segmental artery, as well as the left upper lobe lingular superior segmental artery (Figure 3b).
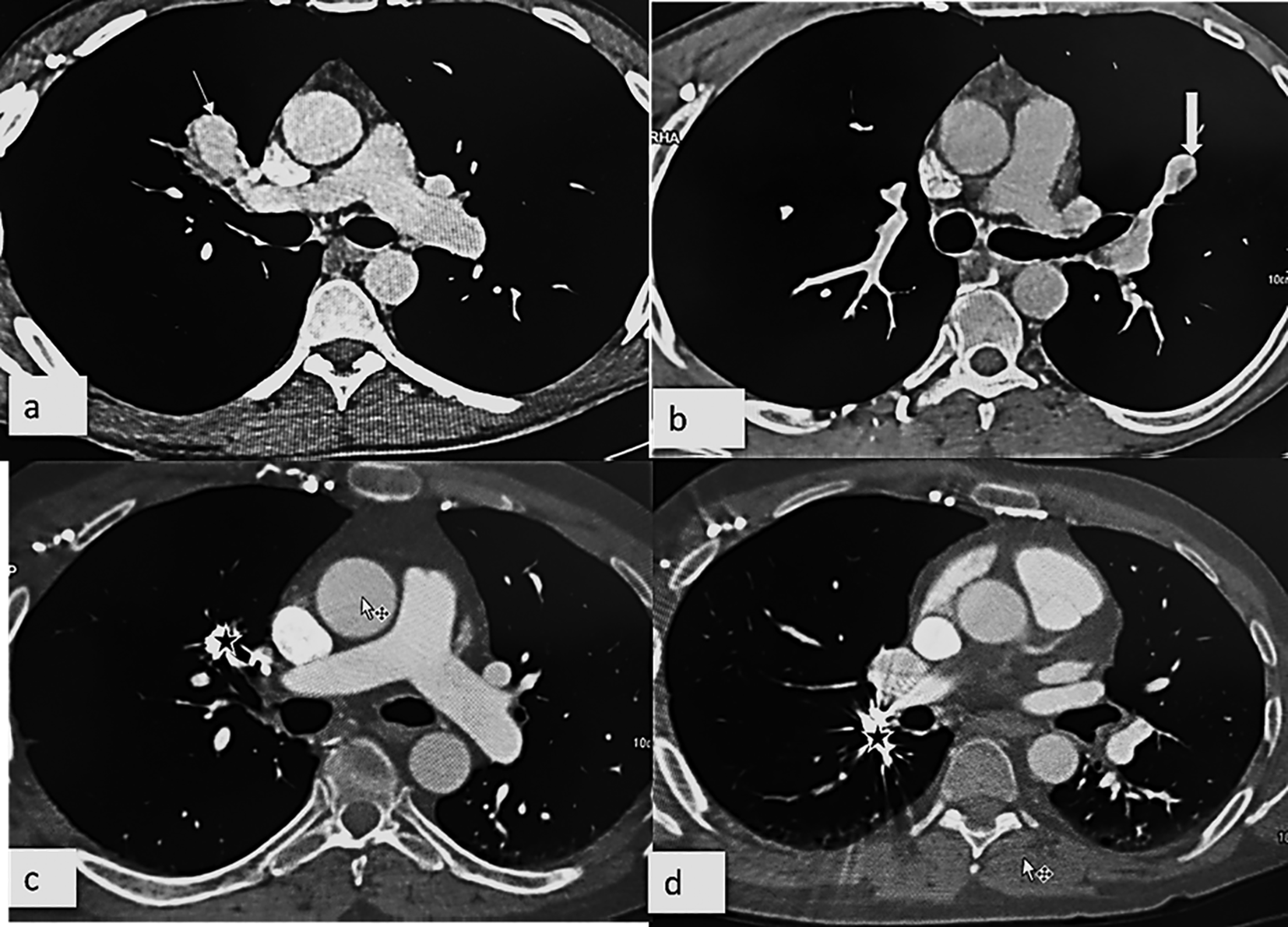
A cystic air-filled formation in the right lower lobe antero basal segment close to the embolization coils was also found. The patient had an embolization of the aneurysms of the right upper lobe ventral segmental and the right lower lobe apical segmental arteries partially thrombosed aneurysms (Figure 3c, d).
During his last admission, he received three methylprednisolone (1g) pulses followed by oral prednisone (40 mg/day), IV periodic pulses of cyclophosphamide (1500 mg/m2 every four weeks during six months) in addition to oral anticoagulation (2 mg of acenocoumarol once daily per os).
The patient has since been lost to follow-up.
This was a 21-year-old North-African university male student who presented to our department seven months after being diagnosed with BD, with recurrent bipolar aphthosis associated with fever in the context of treatment noncompliance. Physical examination showed facial and neck edema, pseudo-folliculitis lesions and prominent superficial thoracic venous collaterals. Vital signs were unremarkable.
First-line laboratory tests including complete blood count, basic metabolic panel, liver and thyroid functions tests, coagulation panel and urinalysis were within normal range except for a hemoglobin concentration of 9 g/dl without particular challenge reported.
Several cardiac masses were detected by transthoracic and transesophageal echocardiography, including two in the right ventricle and one in the right atrium that protruded across the tricuspid valve.
Chest CT scan showed a brachiocephalic vein blockage and several thrombi in the right atrium and the right ventricle, and also in the superior vena cava.
Several pulmonary infarcts and bilateral pulmonary emboli were present in the lower lobes of the lungs. In the right basal segmental artery, a 14 mm diameter aneurysm was observed. Considering the clinic-radiological data, the established association with BD and following the thorough exclusion of other potential differential diagnoses such as Takayasu arteritis, systemic lupus erythematosus, and various connective tissue diseases, the diagnosis of HSS was made.
The patient was admitted on the first day of presentation and given three daily doses of methylprednisolone (1mg/kg/day) based on an initial high suspicion of HSS. A total of 72 h after admission, laboratory tests ruled out the differential diagnosis and the oral corticosteroid was followed by oral prednisone (30 mg/day) over three weeks, in addition to anticoagulation (acenocoumarol 3 mg/day per os during nine months) and monthly cyclophosphamide pulses (2000 mg/m2 during six months), associated with colchicine (1mg/day during nine months).
At nine months follow-up, the patient’s symptoms had improved. CT scan showed no evidence of the previously mentioned thrombosis, as well as the regression of the pulmonary aneurysm, indicating a halt in the disease process with resolution of symptoms.
A 55-year-old North-African daily laborer male patient with unremarkable medical history except for a chronic cough, presented to the emergency department with a pain and swelling of the left leg.
Physical examination revealed few folliculitis lesions on the back and edema of the left leg that was related to a deep vein thrombosis (DVT), confirmed by the doppler ultrasound.
Chest radiography demonstrated a widened superior mediastinum. Further investigation with chest CT scan showed an aneurysm of the ascending aorta of 48 mm.
First-line laboratory tests including complete blood count, basic metabolic panel, liver and thyroid functions tests, coagulation panel, erythrocyte sedimentation rate and urinalysis, rheumatoid factor, antinuclear antibody and immunoglobulin levels were not in favor of thrombophilia or immunological abnormalities. HSS diagnosis was established on the basis of an aortic aneurysm and peripheral venous thrombosis with clinical findings consistent with BD.
Therefore, the patient received three daily pulses of methylprednisolone (1mg/kg/day IV) followed by oral prednisone (40 mg/day) with gradual and monitored decrease in dosage over 6 months, and monthly pulses of cyclophosphamide (2000 mg/m2 IV during six months) relayed by oral azathioprine (2 mg/kg/day) associated with colchicine (1 mg/day per os). The patient also received three months of oral anticoagulation (acenocoumarol 2 mg/day per os).
A follow-up thoracic CT scan performed in the same hospital at six months revealed a regression of the ascending aorta aneurysm.
A 54-year-old North African medical professional male patient was referred to our department after an incidental discovery of an aneurysmal thrombosed postero-basal pulmonary artery during a thoraco-abdomino-pelvic CT scan. This scan was conducted for anatomical kidney assessment in the context of repetitive urinary tract infection. At that time, the patient did not have any relevant personal of family medical history.
The patient also had a history of neglected bipolar aphthae (buccal and genital). Therefore, the patient was admitted to the internal medicine department to assess those lesions. At admission, physical examination was unremarkable except for genital scars. Echocardiography showed a mild circumferential pericardial effusion (4 mm).
A CT angiography of the aorta was subsequently performed two days after admission and showing a thrombosed saccular aneurysm of two right medial basal sub-segmental pulmonary arteries. BD was then retrospectively diagnosed and the treatment started during the third day of admissions based on three daily pulses of IV methylprednisolone (1 g) and a first pulse of a six-monthly cyclophosphamide 1 g treatment protocol were prescribed.
The patient was discharged a week later with oral prednisone (1 mg/kg/day) and colchicine (1 mg/day) and was monitored as an outpatient. Three months later, the patient presented to our tertiary care center with bilateral red swollen legs. At presentation, cutaneous examination revealed bilateral calves swelling, pitting edema and localized pain along vein distribution.
Compression ultrasonography confirmed the diagnosis of bilateral deep venous thrombosis (DVT) of the anterior right tibial vein, and posterior left tibial vein, leading to the diagnosis of HSS. Anticoagulant therapy (acenocoumarol 2 mg/day orally) was then initiated in addition to a pulse of IV methyprednisone (500 mg) and cyclophosphamide (1g). The patient was then discharged and is still being monitored.
A 21-year-old North-African male university student patient presented to our department with massive hemoptysis and dyspnea. He was diagnosed with BD at 16 years of age and treated with colchicine 1 mg/day.
At presentation, his vital signs were within normal limits. On physical examination the patient was pale and had genital scars with oral aphthae and pseudo-folliculitis over his thighs.
At blood examination, he had an elevated C-reactive protein (55 mg/dl) and erythrocyte sedimentation rate (ESR) (82 mm at 1 h). Management consisted of urgent intubation and blood transfusion. Chest CT scan with contrast revealed three aneurysms of the right lateral basal segmental branch and one aneurysm of the posterior segmental branch of the right upper lobe, as well as two aneurysms of the apical posterior segmental branch of the left upper lobe.
A right lower lobe infiltrate consistent with blood was identified, and extravasation of contrast into the lung parenchyma was observed.
Embolization of the right lateral basal segmental branch was successfully performed. Afterwards, the hemoptysis ceased and the patient was extubated. Then, he benefited from three days of methylprednisolone IV pulses (1 g each) and 1 g of cyclophosphamide pulse part of a six-month treatment protocol.
The patient was then discharged home with oral prednisone 1 g/kg/ day and colchicine 1mg/day.
Six months after discharge, the patient developed intermittent hemoptysis with approximately 10 cc per day for three weeks. He admits being non-compliant to his treatment. His records showed that he received only one immunosuppressant pulse.
On physical examination, the patient exhibited right heart failure signs (distension, positive hepato-jugular reflux). A repeat contrast chest CT showed eruption of two new aneurysms along with the previous findings and a left pulmonary artery thrombus.
An elective right lower lobectomy was performed, and anticoagulation treatment was initiated. The patient was also given immunosuppressant therapy (a second six-month cyclophosphamide treatment protocol), along with oral prednisone 1 g/kg/day and colchicine 1 mg/day.
Finally, he was discharged home and did not report any other episode of hemoptysis. He is still being monitored.
HSS is a rare disorder with fewer than 90 cases reported to date in the literature. It was firstly described in 1959 by Dr. John Patterson Hughes and Peter George Ingle Stovin in four male patients.1 This disease is generally characterized by the combination of multiple pulmonary artery (PA) aneurysms and deep vein thromboses (DVT).3
Current literature lacks formally established diagnostic criteria due to the scarcity of this syndrome. Although many hypotheses have been suggested to explain the exact etiology and pathogenesis of HSS, vasculitis remains the most relevant explanation6 similar to that involved in BD. In fact, HSS has been considered as “the cardiovascular manifestation of Behçet’s disease”,7 “incomplete Behçet’s”8 “rare case of Behçet’s disease”,9 and “variant of BD”,10 as both HSS and BD may develop PA aneurysms.11–13
An additional suggested way in which this disease operates is through autoimmune vasculitis inducing a hypercoagulability state.14 This increased tendency to clot may lead to preexisting blood becoming a focal point for bacteria when the patient contracts an infection. Consequently, those thrombi can transform into septic emboli, ultimately causing the characteristic PA aneurysms accentuated with the disease.2 Angiodysplasia has also been suggested as playing a key role in the pathophysiology of this syndrome.15
The course of this condition has been described as following three phases: initial thrombophlebitis, subsequent development of PA and/or bronchial aneurysms, and eventual rupture of the aneurysm, which can result in hemoptysis.
Using the terms “Hughes-Stovin syndrome,” we conducted searches on Google Scholar and PubMed. We looked at all of the relevant case reports and case series, excluding articles without patient information and studies on animal subjects, from the inception date to March 2023.
We identified 88 potentially relevant articles and we excluded 10 after going over the titles, abstracts and full texts. The following articles were excluded: exclusive review articles, articles with insufficient patients’ information, and articles about animal subjects. The results are summarized in Table 2.
The demographic characteristics of a total of 78 patients including our 5 patients were 64 males and 14 females. A male predominance was noted in the literature and all of our patients were male. The mean age was 32.1 (range 11–55 years), however the age of one patient wasn’t mentioned. In the present study, mean age was 40.4 (range 21–55 years).
This syndrome usually affects young adults.15 A total of 12 patients were identified as having BD associated to HSS and 14 others had some of the classical features of BD without fulfilling the diagnostic criteria. However, in the current case series, all the patients presented BD features.
At disease onset, patients may present with DVT, right heart failure, superficial thrombophlebitis, hemoptysis, headache, diplopia and seizures.2,16
All these clinical features are also encountered in many other diseases indicating the diagnostic difficulties. Consequently, special attention should be given to clinical or radiological manifestations allowing the differentiation.
Moreover, the diagnosis might be fortuitous, as we described in the case number 4. Fortunately, for the first time since its initial report in 1959, a promising start has been made by the Hughes-Stovin Syndrome International Study Group (HSSISG), creating a comprehensive reference atlas of computed tomography pulmonary angiography (CTPA) images, a guide defining the wide spectrum of CTPA findings that have been observed in HSS.17,18
Triggianese et al. presented an interesting approach linking HSS to HLAB51 in their case report, which is in contradiction with Manole et al.’s work suggesting that HSS is a mainly vascular disorder associated with certain minor genetic variations that can interfere with proper vascular function.4,5
These assumptions need further investigation but are hindered by the scarcity of patients. From the anatomical perspective, aneurysms may be single, multiple, unilateral or bilateral.19
They can arise anywhere in the central circulation as shown in our case series; however, the pulmonary region remains preferentially affected. In fact, our review indicates that nearly two thirds of cases presented with pulmonary aneurysms.
Thrombosis observed in HSS involves large vessels including the vena cava, jugular, iliac, femoral and the brachiocephalic veins (as we reported in the case number 2), as well as cardiac chambers and dural sinuses.6,8,20–23
Being an exceedingly rare disorder of unknown etiology, no randomized case control trials are available to help select the most appropriate and effective treatment for HSS. Recent literature reviews reported treatment success with corticosteroids and/or immunosuppressants. Cyclophosphamide is the most commonly used immunosuppressant followed by azathioprine.24 These treatments are considered by almost all authors as a first line medical treatment option of HSS.6,12 All of our patients were treated with corticosteroids, cyclophosphamide and oral anticoagulation.
The use of anticoagulants and thrombolytic agents remains controversial; they increase the risk of fatal hemorrhage while conferring a beneficial effect within a pre-embolic or embolic state. Consequently, clinicians should carefully consider case by case scenarios when recommending these therapies. Inferior vena cava (IVC) filter placement may be of interest in extreme cases.5
Anti–tumor necrosis factor α (TNF-α) presents a new medical treatment option with promising perspectives. It provides a stable disease remission and cases of complete resolution of pulmonary aneurysms have been reported.25
Surgical resection is still described as the gold standard treatment when the patient presents with severe or recalcitrant hemoptysis.26 However, surgery is not considered in cases of bilateral, extensive PA aneurysms. Thus, transcatheter arterial embolization is regarded as a promising alternative as it is less invasive and allows selective treatment of multiple and/or bilateral aneurysms,15 and prevents massive hemoptysis.
HSS remains a diagnostic and therapeutic dilemma due to its uncommon occurrence and limited understanding of its pathogenesis.
Through our review of the literature and presentation of our case series, we have underscored the importance of considering HSS as a differential diagnosis in patients presenting with PAA and DVT. The association with BD and the exclusion of other potential differential diagnoses such as large-vessel arteritis, Wegener’s granulomatosis, systemic lupus erythematosus, and connective tissue diseases are crucial in making an accurate diagnosis.
The therapeutic management of HSS poses significant challenges. Our case series highlights efficient approaches undertaken, ranging from medical management alone based on corticosteroids, immunosuppressants and anticoagulation, to embolization and embolectomy in the most extreme cases, underscoring the individualized nature of therapeutic decisions.
Further research is warranted to enhance our understanding of the underlying pathogenesis, optimal diagnostic strategies, and treatment modalities for HSS. In order to improve patient outcomes and create evidence-based guidelines, collaboration between healthcare professionals is essential. Multidisciplinary teams should include internists, rheumatologists, cardiologists, radiologists, and surgeons.
By increasing awareness and understanding of this rare syndrome, we aim to improve early recognition, prompt management, and ultimately enhance the prognosis for patients affected by HSS.
| Number of cases | Gender | Age | Findings | BD features | |
|---|---|---|---|---|---|
| El Jammal, Gavand,27 2019 | 1 | M | 19 | +- | |
| Bin Pervez, Iqbal,28 2020 | 1 | M | 31 | - | |
| Dahi, Keese,29 2019 | 1 | M | 22 | - | |
| Chalazonitis, Lachanis,7 2009 | 1 | M | 18 | - | |
| Keskin, Polat30, 2020 | 1 | F | 28 | - | |
| Pankl, Meraldi,31 2015 | 1 | M | 41 | - | |
| Valdés-Corona, Kimura-Hayama,32 2020 | 1 | M | 19 | - | |
| Silva, Escobar,33 2013 | 1 | M | 25 | - | |
| Emad, Ragab,34 2019 | 1 | M | 35 | - | |
| Takaori, Kitazawa,35 1989 | 1 | M | 31 | + | |
| Bawaskar, Chaurasia,36 2019 | 1 | M | 21 | - | |
| Demirkan and Gültekin,37 2018 | 2 | F | 37 | +- | |
| M | 33 | +- | |||
| Nishi, Myou,9 1993 | 1 | M | 27 | +- | |
| Riantawan, Yodtasurodom,38 1999 | 1 | M | young | - | |
| Jambeih, Salem,39 2015 | 1 | F | 48 | - | |
| Kechida, Yaacoubi,40 2017 | 1 | M | 55 | + | |
| Choh, Jehangir,41 2011 | 1 | M | 40 | - | |
| Al-Jahdali,19 2010 | 1 | F | 23 | +- | |
| Tzilalis, Vourliotakis,42 2011 | 1 | M | 18 | - | |
| Kim, Kim,43 2010 | 1 | M | 24 | - | |
| Villié, Noël,44 2016 | 1 | M | 28 | + | |
| Herb, Hetzel,45 1998 | 1 | M | 25 | - | |
| Roberts, Jimenez,46 1982 | 1 | M | 12 | - | |
| Teplick, Haskin,47 1974 | 1 | F | 25 | - | |
| Thrombus, Tymko,48 2020 | 1 | M | 25 | - | |
| Abdelbary, El-Masry,49 2016 | 1 | F | 35 | - | |
| Fischer, Korman,50 2005 | 1 | M | 49 | - | |
| Ghirardo, Pastore,25 2019 | 1 | M | 17 | - | |
| Al-Zeedy, Jayakrishnan,51 2015 | 1 | M | 53 | - | |
| El Aoud, Frikha,52 2014 | 1 | F | 42 | +- | |
| Robinson, Miller,53 2018 | 1 | M | 21 | - | |
| Bennji, du Preez,54 2017 | 1 | M | 34 | + | |
| Nishi, Myou,9 1993 | 1 | M | 27 | + | |
| Fabi, Lami,55 2017 | 1 | M | 12 | + | |
| Meireles, Sobrinho-Simões,56 1981 | 1 | M | 33 | - | |
| Durieux, Bletry,57 1981 | 4 | M | 35 | + | |
| M | 32 | + | |||
| M | 35 | + | |||
| M | 31 | - | |||
| Margolesky, Tornes,58 2015 | 1 | F | 38 | - | |
| Mahfoudhi and Turki,59 2015 | 1 | M | 27 | - | |
| Kinjo, Tanaka,60 1978 | 1 | F | 37 | - | |
| Emad, Ragab,8 2007 | 2 | M | 26 | - | |
| M | 16 | - | |||
| Kindermann, Wilkens,61 2003 | 1 | F | 50 | - | |
| Tsai, Lu,26 2005 | 1 | M | 34 | - | |
| Kim, Oh,62 2007 | 1 | M | 45 | - | |
| Higuchi, Kitamura,63 1969 | 1 | M | 37 | - | |
| de Vries, Koppelman,64 2011 | 1 | M | 11 | - | |
| Yagi, Yamagishi,65 2001 | 1 | M | 32 | +- | |
| Lee, Noh,23 2008 | 1 | M | 48 | - | |
| Amezyane, Bassou,66 2010 | 1 | F | 28 | +- | |
| Jaramillo, Gómez-Bueno,67 2015 | 1 | M | 47 | - | |
| Balci, Semelka,68 1998 | 1 | M | 41 | - | |
| Alí-Munive, Varón,69 2001 | 1 | M | 37 | - | |
| Weintraub, DeMayo,6 2001 | 1 | M | 22 | - | |
| Ketchum, Zamanian,70 2005 | 1 | M | 49 | - | |
| Bowman and Honey,13 1990 | 1 | M | 27 | +- | |
| Kopp and Green,71 1962 | 1 | M | 30 | - | |
| Kably and Reveron,72 2015 | 1 | F | 41 | - | |
| Khalil, Parrot,20 2006 | 1 | M | 42 | - | |
| Lee, Hoon,73 2014 | 1 | F | 19 | +- | |
| Roberts, Jimenez,46 1982 | 1 | M | 12 | - | |
| Wakamiya, Fujiwara,74 1991 | 1 | F | 54 | - | |
| Hamdy et al.,75 2020 | 1 | M | 34 | - | |
| Triggianese et al.,5 2021 | 1 | M | 33 | +- | |
| Azhar et al.,16 2022 | 1 | M | 26 | ||
| Cole, Mandava,14 2022 | 1 | M | 28 | +- | |
| Manole4 et al. 2023 | 1 | M | 35 | - | |
| Moussa et al.,76 2021 | 1 | M | 26 | - | |
| Sofi et al.,10 2022 | 1 | M | 25 | +- | |
| Vyas et al.77 | 1 | M | 27 | - |
The Declaration of Helsinki-Ethical Principle for Human Subjects in Medical Research guided the conduct of this paper.
Written informed consent was obtained from all participants prior to their inclusion. The patients also provided written informed consent for the publication of their clinical details and images.
The illustrations and scans utilized in this case series paper are permitted for utilization, with all identifying individual features eliminated. This was done to protect the privacy and confidentiality of the individuals involved and to comply with ethical standards in scientific research. All images presented in this paper have been edited to remove any identifying information.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Rheumatology, Vasculitis, RA, SLE
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Aug 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)