Keywords
Gluten-free foods, Carotenoids, Extrusion, High-protein quinoa flour, Plant-based snacks, Polyphenols, Antioxidant properties
Gluten-free foods, Carotenoids, Extrusion, High-protein quinoa flour, Plant-based snacks, Polyphenols, Antioxidant properties
According to the Good Food Institute, the majority of plant-based foods have a high demand in the market because consumers are seeking healthier and environmentally friendly options. In this category of plant-based foods, a food technology trend for 2023 will be to produce matrices with higher protein content. In this regard, products such as quinoa, amaranth, sacha inchi, and others are presented as alternatives for the production of vegan, gluten-free foods with high protein and bioactive compound contents.1–4 Quinoa has a protein content of 18%,5 and its digestibility after thermal and high-pressure treatment is above 90%.6 On the other hand, sacha inchi has a protein content of 25–30%7 and a digestibility of 41% after extrusion.8 Studies on these types of plant matrices show that processes like extrusion can improve the availability of nutrients such as protein, certain compounds affecting antioxidant activity, and the content of polyphenols.9–11
Within the food category, extruded snacks are an alternative with a market size of $48.3 billion in 2019, projecting a compound annual growth rate (CAGR) of 4.4%. Additionally, the launch of gluten-free products increased by 73% and vegan products by 196%, partly attributed to policies and claims of health properties associated with the food products offered to consumers.12 Therefore, it is necessary to promote research to evaluate the influence of new vegetable sources in the elaboration of snacks, the conditions for their processing and their physical, chemical and sensory characteristics. This technology is widely used to improve their nutritional quality, with an emphasis on protein content.13 In this type of food, textural properties such as hardness, shear resistance, tensile strength, freshness, and crunchiness are crucial for controlling processing operations and achieving the desired quality attributes of the finished product and consumer acceptability.14
According to Ref. 15, these foods can contribute to increased energy intake and body weight gain in adults, considering that they are consumed at any time of the day during leisure activities (such as in front of the television or computer). Providing healthy snacks is an attractive alternative for the food industry. Studies in Europe have considered quinoa as part of a variety of protein-rich crops that have the potential to diversify global production systems focused on a limited number of species. This would help diversify diets and offer the possibility of generating new plant-based feeding alternatives that contribute to reducing greenhouse gas emissions and negative impacts on the environment.16 On the other hand, sacha inchi presents beneficial effects for various activities such as neuroprotective modulation, dermatological effects, antidyslipidemic effects, antioxidant properties, anti-inflammatory effects, antiproliferative effects, and antitumor effects. These benefits are associated with its bioactive compounds, particularly essential fatty acids, proteins, and phytochemicals.4
Fourier transform infrared spectroscopy – attenuated total reflectance (FTIR–ATR) analysis allows for the identification of the position, intensity, and shape of infrared peaks,17 which can reveal chemical bonds and quantify chemical structures of macromolecules such as carbohydrates, lipids, fiber, moisture, bioactive compounds, antinutrients, and other compounds of interest. This analysis helps assess the quality of the food matrix.18–21 Several chemical assays have been designed to measure the molecular or cellular level capacity for radical elimination, reducing power, and other specific attributes of antioxidants, as well as the overall inhibition of oxidation in foods and more complex biological systems.22 In this study, we measured antioxidant values using the, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), and ferric reducing antioxidant power (FRAP) methods.
The objective of this study was to evaluate the inclusion of high-protein quinoa and sacha inchi cake in extruded and puffed snacks, resulting in a high-protein food with bioactive compound content, to offer vegan and gluten-free food alternatives.
The cereals used in this study were provided by “SEGALCO” Company S.A.S. Popayán, Colombia. Quinoa was ground in a quinoa polishing machine (500-T, Mavimar, Colombia) with a processing capacity of 60 kg/h. This grain abrasion process allows obtaining a high-protein quinoa flour (HPQF). Sacha inchi almond was received in almond form and then subjected to a pressing process (CGLDENWALL, store model K28, Shanghai, China). The 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) diammonium salt, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), and ferric reducing antioxidant power (FRAP) were acquired from Thermo Fisher Scientific (Massachusetts, USA). Ethanol (96%), acetone, hydrochloric acid, petroleum ether (98%), Butylhydroxytoluene (BHT), and Folin & Ciocalteu’s phenol reagent were obtained from Commercial Outsourcing (Manizales, Colombia).
The snack pellets were prepared according to the formulations shown in Table 1, treatments T1, T2 and T3 correspond to the different inclusions, which consisted of whole rice flour (WRF), HPQF, and sacha inchi flour (SIF) using a twin-screw industrial extruder (CY65-II TWN SCREW EXTRUDER, Qingdao, China). T1: WRF 55%, SIF 25% and HPQF 20%; T2: WRF 45%, SIF 25% and HPQF 30%; T3: WRF 25%, SIF 25% and HPQF 50%. The mixtures were moistened to a 32% moisture content by spraying water and continuous mixing. Then, the extrusion-cooking process was initiated at temperatures of 60°C, 80°C and 110°C, with a screw rotation speed of 200 rpm, and a nozzle diameter of 3 mm. The snack pellets were dried at 100°C for 30 minutes. Afterward, these pellets were moistened to a moisture content of 14%, cut to a length of 5 mm, and inflated using a machine (RICE CAKE MACHINE SYP4006, BUCHEON-SI, GYEONGGI-DO, Korea). The blowing conditions were a temperature of 230°C, a pressure of 100 bar, and a heating time of 10 seconds.
The proximate composition of the macronutrients protein, lipids, dietary fiber, ash and moisture of taro and sacha inchi flours was measured according to the methods proposed by the AOAC (Association of Official Analytical Chemists, 1990)15 and the carbohydrate content was estimated by difference. The details of the method are explained in the protocols uploaded to the repository.23
Protein
We weighed 0.2 g of sample plus 1 g of Kjeldahl catalyst, added 10 mL of sulfuric acid and heated until the samples were completely clear and translucent, free of organic matter, in the laboratory Kjeldahl digester (Raypa MBC-6/N, Spain). After this process they were cooled to room temperature, then passed to the Raypa distillation unit, and each sample was titrated with 0.1 N HCl.
Lipids
To start the lipids determination, 1 g of sample was weighed into the extraction cartridges and 80 mL of petroleum ether was added, and this was transferred to the rack of the laboratory Soxhlet and Randall extractor (SX-6MP, RAYPA, Spain). After the extraction time the samples were placed in an oven at 60 °C for 1 hour to remove the remaining ether.
Fiber
To start the fiber measurement, 1–2 g of sample were transferred to the laboratory fiber extractor (F-6P Fibertest, Spain). Then, 150 mL of 0.255 N H2SO4 was added to an Erlenmeyer flask, the heating knob was adjusted to boiling point and left boiling for 30 min. After this time, it was filtered and washed with distilled water three times using 30 mL of water each time.
The FT-IR spectra of the flours and extrudates were evaluated using an FTIR spectrometer (IS50 Nicolet, Thermo Scientific, USA) with an ATR accessory. The intensity evaluation was performed on the spectrum after baseline subtraction, applying a 10-point smoothing, and normalizing the data from 0 to 1 in Origin v18 software. The height of the transmittance bands was determined from their baseline. The specific areas of interest studied included protein (amide I, II groups), protein secondary structure in the IR regions of approximately 1200–1800 cm-1. Additionally, the crystalline regions of starch were approached from 1200 to 800 cm-1 to evaluate changes in their relative intensities. The amide I region (1700–1600 cm-1) located within the protein’s spectral range was subjected to second derivative analysis to observe overlapped peaks in that region, corresponding to β-sheets and α-helix.
The extraction and analysis of EPC were based on the method described in Ref. 24 with slight modifications. Approximately 2 g ± 0.0500 g of sample sieved through a 200 μm sieve were weighed. Two extractions were performed, the first with ethanol/H2O (80/20, plus 1% formic acid) and the second with acetone/H2O (70/30), the supernatant from both extractions was combined and brought to a final volume of 20 ml with deionized water. The extract was kept at -80°C for colorimetric determination by the Folin-Ciocalteu reaction or evaluation of antioxidant capacity. The sediment was also kept at -80°C for the determination of hydrolysable phenolic compounds (HPC).
The extraction and analysis of HPC were based on the methods described by Ref. 24, with slight modifications. Approximately 0.8 g ± 0.0050 g of sediment from EPC was weighed, and then 10 mL of methanol/H2SO4 (90/10) was added. The tubes with the sample and solution were left for 22 hours at 85°C with magnetic stirring. The extract was kept at -80°C for colorimetric determination by the Folin-Ciocalteu reaction.
The content of EPC and HPC was expressed in mg of gallic acid equivalents per gram (GAE/g), of dry matter. All analyses were performed in triplicate.
To measure the antioxidant properties, EPP extracts were used.
ABTS
In a test tube, 4 mL of ABTS solution were placed and covered completely with aluminum foil. To initiate the process, 135 μL of standard solution was added and then mixed in a vortex for 5 s. The blank reagent consisted of 4 mL of acetate buffer and 135 μL of ethanol. The zero point was mixed with 4.5 mL of ABTS solution and 135 μL of ethanol. The test tube was closed and allowed to react for 30 min, and then the absorbance was measured at a wavelength of 729.7 nm using a UV-Vis spectrophotometer (GENESYS™ 10S Thermo Scientific, USA).
DPPH
In a test tube, 3.9 mL of DPPH solution and 100 μL of standard solution were applied to initiate a reaction by vortex agitation for 5 s. The blank reagents (control) were carried out with ethanol. The zero point was adjusted by adding 3.9 mL of DPPH solution and 100 μL of ethanol. The sample was covered for 30 min to initiate the reaction, and then the absorbance was measured at 517 nm using a UV-Vis spectrophotometer (GENESYS™ 10S Thermo Scientific, USA).
FRAP
In a test tube, 1.8 mL of solution containing (2.5 mL of 0.01 M TPTZ, 2.5 mL of 0.02 M FeCl3, and 25 mL of 0.3 M sodium acetate buffer pH 3.6), 180 mL of distilled water, and 60 μL of sample solution were applied, vortexed for 15 s to initiate a reaction, and then left at 37°C for 30 min. The blank reagents (control) were carried out with distilled water, and then the absorbance was measured at 595 nm using a UV-Vis spectrophotometer (GENESYS™ 10S Thermo Scientific, USA).
The extraction of carotenoid pigments was performed following the methodology described by Refs. 25–27 with modifications, in two stages: solid-liquid extraction and liquid-liquid extraction, of which the first extraction stage was adjusted.
The mechanical properties of the extrudates were determined following the method by Ref. 28 with slight modifications. They were analyzed using a texture analyzer (Shimadzu EZ TEST SM, model 500N-168, Japan). The area under the curve (S; N.mm) and the number of peaks (n) exceeding 1.5 N were calculated from the force-deformation curves and used to calculate the spatial frequency of ruptures (Nsr) (equation 1), average crushing force (Fcr) (equation 2), and crispness work (Wcr) (equation 3).
The color was determined using a Konica Minolta CM-5 Spectrophotometer colorimeter, controlled by SpectraMagic NX software, with illuminant D65 and an observer angle of 10°. The values of luminosity (L*), green/red chromaticity (a*), and blue/yellow chromaticity (b*) were obtained, from which the chromaticity values (C*) (equation 4), hue angle (h°) (equation 5), total color difference (∆E) (equation 6), whiteness index (WI) (equation 7), and browning index (BI) (equation 8) were calculated.
A completely randomized design was employed to assess the incorporation of quinoa in high protein snacks formulation. Three inclusion levels were considered: 20%, 30% and 50%. The response variables included polyphenols, antioxidant activity, carotenoids, texture, and color.
The results were presented as the mean ± standard deviation of triplicate or more experiments, as appropriate. To compare the means, a one-way analysis of variance (ANOVA) was conducted. Significance of differences between means was determined at p < 0.05 by Tukey’s new multiple range test. Statistical data and curves were analyzed using Origin v 18, open-access alternatives that can perform an equivalent function are Oracle SQL, Matplotlib and R.
The following results were analyzed according to the data found in the repository.29
Table 2 presents the proximate composition of the extruded and popped snacks containing HPQF, SIF, and rice.
The protein, lipid, ash, and fiber contents of snacks T1, T2, and T3 significantly increased (p < 0.05) with the inclusion of HPQF, while the carbohydrate content decreased. Quinoa flour contains a higher amount of protein, fiber, and lipids than other flours like rice and corn, which could be the reason for the increase in these macronutrients in the snacks. In previous studies,30 we reported that HPQF contained 30.12 g/100 g of protein, a higher amount than rice flour (9.4 g/100 g) and corn flour (7 g/100 g). On the other hand,31 reported a protein content of 11.45 g/100 g in defatted quinoa flour, higher than other cereals.
According to studies, the removal of pericarp and aleurone layers of grains during milling affects the protein content.32 In this sense, quinoa, due to its size and structure, requires special conditions for milling and dehulling that allow extracting the endosperm without breaking it and obtaining a hyperprotein quinoa flour (HPQF) at a low cost and with reduced environmental impact. Based on this, in this study and our preliminary studies,33 we have proposed obtaining quinoa flour with a protein content close to 30%, allowing inclusions in food production with higher protein contents than those available in the market. Therefore, the addition of SIF is also an alternative that allows obtaining foods with a high protein content. Comparing the protein content of the snacks presented in Table 3 with others made from vegetables, we can observe a higher value in our snacks.34,35
However, it is also important to highlight that the superior nutritional value of proteins from pseudocereals such as quinoa and sacha inchi seed over those from cereals also lies in their balanced composition; they are richer in some essential amino acids, particularly high in lysine, the limiting amino acid in most cereal grains, their essential amino acid balance is excellent due to a wider range of amino acids than in cereals and legumes.36 The sacha inchi cake on its part contains mainly amino acids such as glycine 201–215 mg/g protein, leucine 28–39 mg/g protein, threonine mg/g protein, 48–64 mg/g protein, and isoleucine 30–36 mg/g protein.37 It can be observed that, except for lysine and leucine, this would be meeting the amino acid requirements in the diet recommended by FAO for all age groups, except infants, so the union with quinoa is ideal to obtain the balance of amino acids in the snack.
Fourier transform infrared spectroscopy was used to study the composition of the snacks and to analyze the modifications induced by HPQF inclusions.
Infrared spectroscopy is a physicochemical analytical technique used to identify conformational changes in functional groups and structural properties; however, the interpretation of a spectrum presented in a food sample can be complex due to the interaction and amount of compounds present in the sample, because the signals often overlap, which is why subtle changes are difficult to detect.18 For this reason, in this study, spectral analysis was performed on individual flours in order to detect the effect of the inclusion of the flours in the snack and the extrusion process.
The FT-IR spectra of quinoa, rice and sacha inchi flours and extrudates T1, T2 and T3 are shown in Figure 1. The FT-IR spectra show the three separate zones to analyze starch, proteins and lipids. The bands obtained by FT-IR spectroscopy were explained according to previous literature reported for similar flours.19
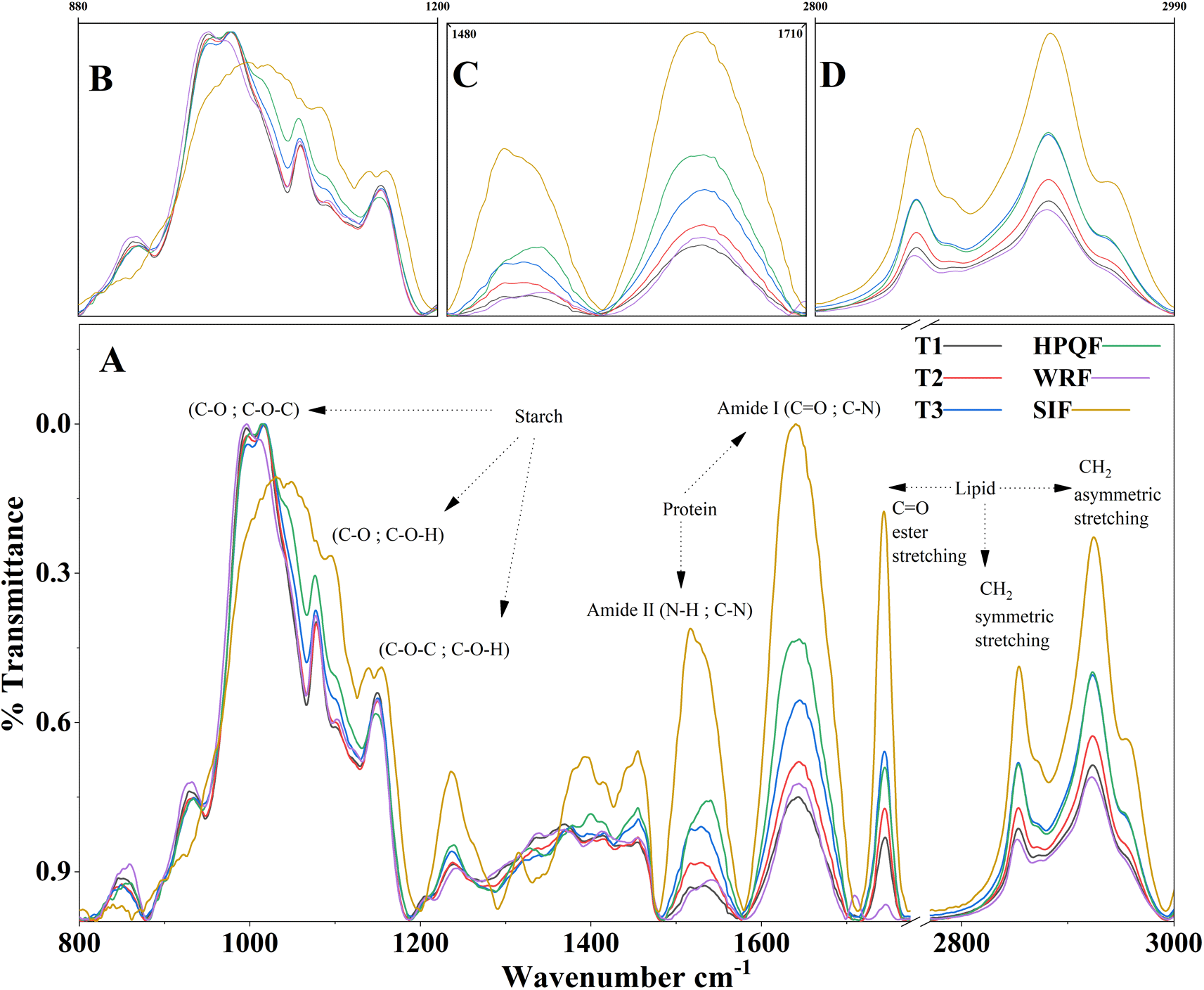
(A) Complete spectra, (B) characteristic starch bands, (C) characteristic bands protein, (D) characteristic bands lips. (T1) treatment 1 with addition of 20% quinoa; (T2) treatment 2 with addition of 30% quinoa; (T3) treatment 3 with addition of 50% quinoa. WRF: Whole rice flour; SIF: Sacha inchi flour; HPQF: High protein quinoa flour.
The wavelength range from 800 to 1200 cm-1 corresponds mainly to the stretching vibrations that can be divided thus C-O, C-O-C; C-O, C-O-H; and C-O-C, C-O-H characteristic in starch (Figure 1B). The IR spectra of the snacks (T1, T2 and T3) and HPQF, WRF in this region are characterized by three main modes with maximum transmittance at 1078, 1014, 996 cm-1. The IR bands at 1078 and 1014 cm-1 are associated with the ordered and amorphous structures of starch, respectively and the band at 999 cm-1 is related to hydrated crystalline samples.18
The main band of raw quinoa flour at 996 cm-1 can be attributed to the contribution of starch and corresponds to the C-O bending of the glycosidic bond. As seen in Table 2, according to the statistical analysis the intensities in the 1078 band were significantly affected (p<0.05) by the addition of HPQF, presenting values of 0.218, 0.3535, 0.3747, 0.3822, 0.3974 and 0.3987 for SIF, HPQF, T3, WRF, T1 and T2, respectively. The intensity is significantly higher for SIF followed by HPQF i.e., for raw flours, this indicates a loss of the ordered structure in the starch fraction, which changes after the extrusion and compression process.
As for the protein analysis, the determination of the secondary structure of this macronutrient is mainly based on the analysis of the amide I band between 1700 and 1600 cm- 1. The peaks of the samples confirmed the typical characteristics of the protein spectrum, with amide I (1643 cm -1) and amide II (1533 cm- 1) (Figure 1C), of the protein samples, arising from specific stretching and bending vibrations of the protein backbone. Similarly, for sacha inchi flour, the maximum range for amide I was 1638 cm -1, whereas for amide II it was 1517 cm -1. The distinct peak in amide I was mainly caused by N-H stretching vibrations, whereas the single peak in amide III was caused by C-N stretching and N-H bending vibrations.38
To reveal a resolution of the molecular components of the proteins, a second derivative analysis on the ATR-FTIR spectra of the middle band I (Figure 2) and a Gaussian curve fitting method were employed to quantify each of the secondary components of the proteins. As shown in Figure 2, the extrusion process caused changes such as denaturation or hydrolysis, unfolding/unwinding of coiled/uncoiled structures, aggregation to form a new conformation and separation to form subunits in an unfolded/unwound manner. A similar situation was presented by Ref. 39, who performed a hydrolysis process through fermentation of oats. The strong vibrations of stretching, bending and the appearance of displaced or new peaks indicate that the quinoa, sacha and rice flours were modified by the extrusion and compression processes.
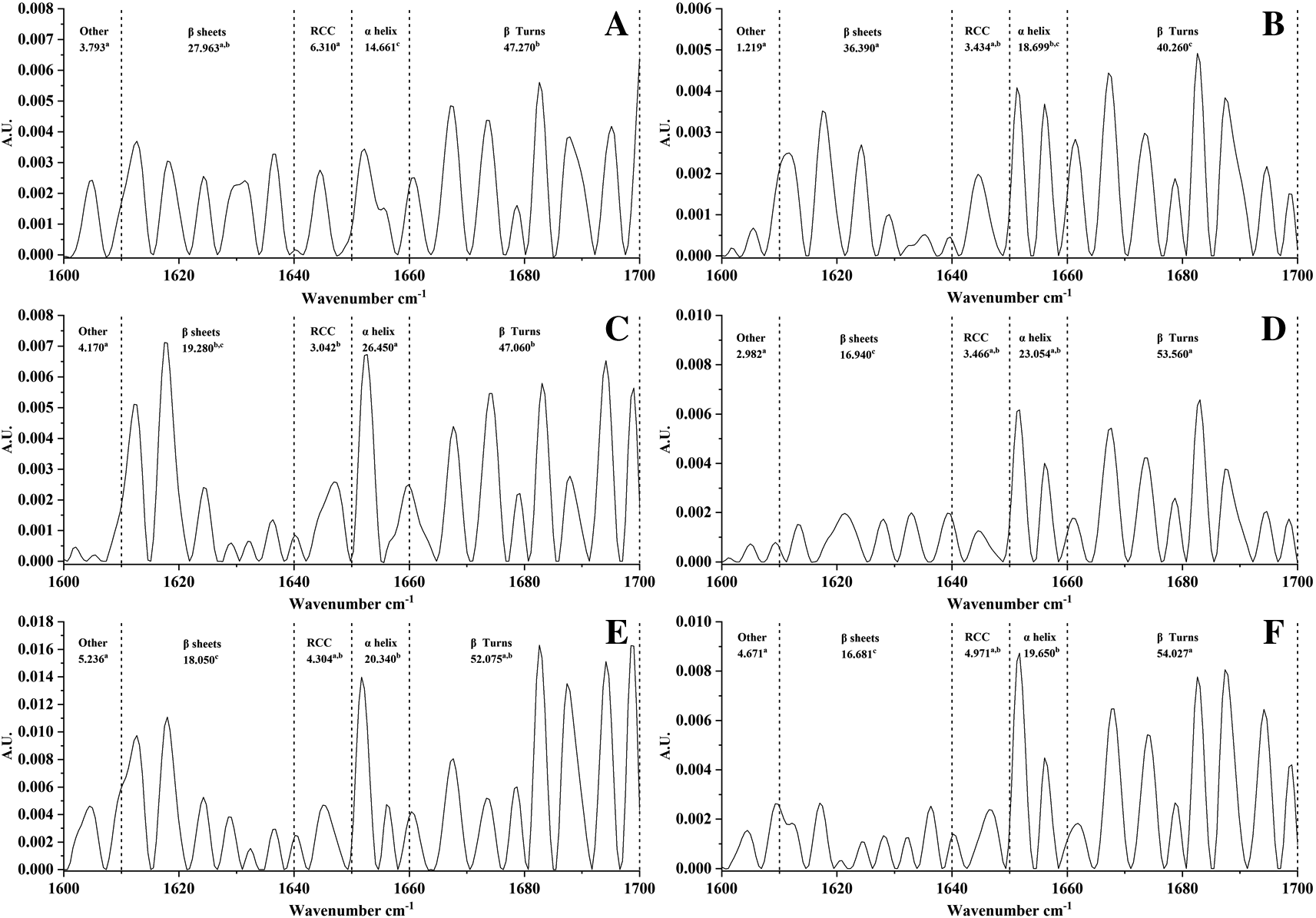
(T1) treatment 1 with addition of 20% quinoa; (T2) treatment 2 with addition of 30% quinoa; (T3) treatment 3 with addition of 50% quinoa. WRF: Whole rice flour; SIF: Sacha inchi flour; HPQF: High protein quinoa flour. Values are ± standard deviation. a, b, c: different letters are significantly different (p < 0.05) between sample.
According to Ref. 40 the region corresponding to amide I is divided into 1651–1660 cm-1 which is assigned to α-helix, between 1660–1700 cm-1 would belong to β-turns, between 1610–1640 cm-1 can be considered as β-sheets and between 1640–1650 cm-1 is assigned to random spiral conformation.
The percentages of the secondary structures of the pure and processed treatments were significantly affected by the addition of quinoa and by the technological process (extrusion and compression). The percentages of the secondary structures of the raw and processed treatments were significantly affected by the addition of quinoa and by the technological process (extrusion and compression). The percentages of the areas can be observed in Figure 2. With respect to the β-sheet conformations, T1 and WRF do not have significant differences and present the highest percentages of 36.39 and 27.96 respectively; T2, T3, and SIF do not present significant differences. Also, random coil conformation (RCC) is statistically different for WRF (6.31%) and HPQF (3.04%), and the other samples do not present significant differences. On the other hand, the α-helix is higher in HPQF (26.45%) statistically different from SIF (20.34%), T3 (19.65%) and WRF (14.66%). Finally, the β-turn presents the largest area in T3 (54.027%) and T2 (53.56%) without significant differences, WRF (47.27) and HPQF (47.06%) were classified in the same group, i.e., they do not present significant differences and T1 (40.26%) presented the lowest value.
According to the above, the different conformations represent the changes that occur in the protein structures due to the flour mixture and the transformation process. The results of the FTIR analysis confirmed that the proteins of the flour and processed sample presented normal characteristics of a protein spectrum.
The lipid bands of the raw and extruded flours were at 1745, 2853 and 2923 cm-1 (Figure 1B), corresponding to the C=O, CH2 symmetrical stretching and CH2 asymmetrical stretching groups, respectively. As can be seen in Table 2, in the 1745 band, the highest intensity was recorded by the sacha cake flour followed by T3, T2 and HPQF; these last two do not present significant differences and recorded at 1745, 2853 and 2923 cm-1, which correspond to the C-H vibration.
According to the results presented in Figure 3A, the EPC in the extrudates varied significantly (p < 0.05) from 1.29, 1.95, and 3.05 mg of GAE/g for T1, T2, and T3, respectively, with the highest being the one containing 50% quinoa. Similarly, the HPC in extrudates varied significantly (p < 0.05) between 11.08, 12.45, and 14.16 mg GAE/g for T1, T2, and T3, respectively. The highest values were presented by the mixtures with a higher content of quinoa. Polyphenols are active compounds produced during the secondary metabolism of plant matrices, containing one or more aromatic rings and hydroxyl groups in their structure. They exhibit properties such as antioxidant, anti-inflammatory, antimicrobial, anti-aging, cardiotonic, diuretic, laxative, hypoglycemic, and anticorrosive.41 According to Ref. 42, quinoa contains the following polyphenols: rutin, vanillic acid, ferulic acid, kaempferol, quercetin derivatives, p-coumaric acid, daidzein, caffeine, caffeic acid, pinocembrin, apigenin, and pinocembrin. In this type of plant-based matrices, polyphenols are divided into two types, EPC and HPC. The former is found in free form in the matrix and requires extraction with water or polar organic solvents such as methanol, ethanol, acetonitrile, acetone, or their water mixtures for their identification. The latter requires hydrolysis with acid or alkali to release phenolic compounds such as ferulic acid and lignans.43 In previous studies,6 we have shown how extrusion contributes to the release of phenolic compounds in snacks made from quinoa. If we compare those studies with the current one, the addition of sacha inchi may increase the content of both EPC and HPC, as the SI seed contains a level of polyphenols that can vary between 6.46–8.00 mg of GAE per gram of seed on a wet basis. Although these phenolic compounds can be altered by heat treatments and pressure, they are still present in the matrices.44
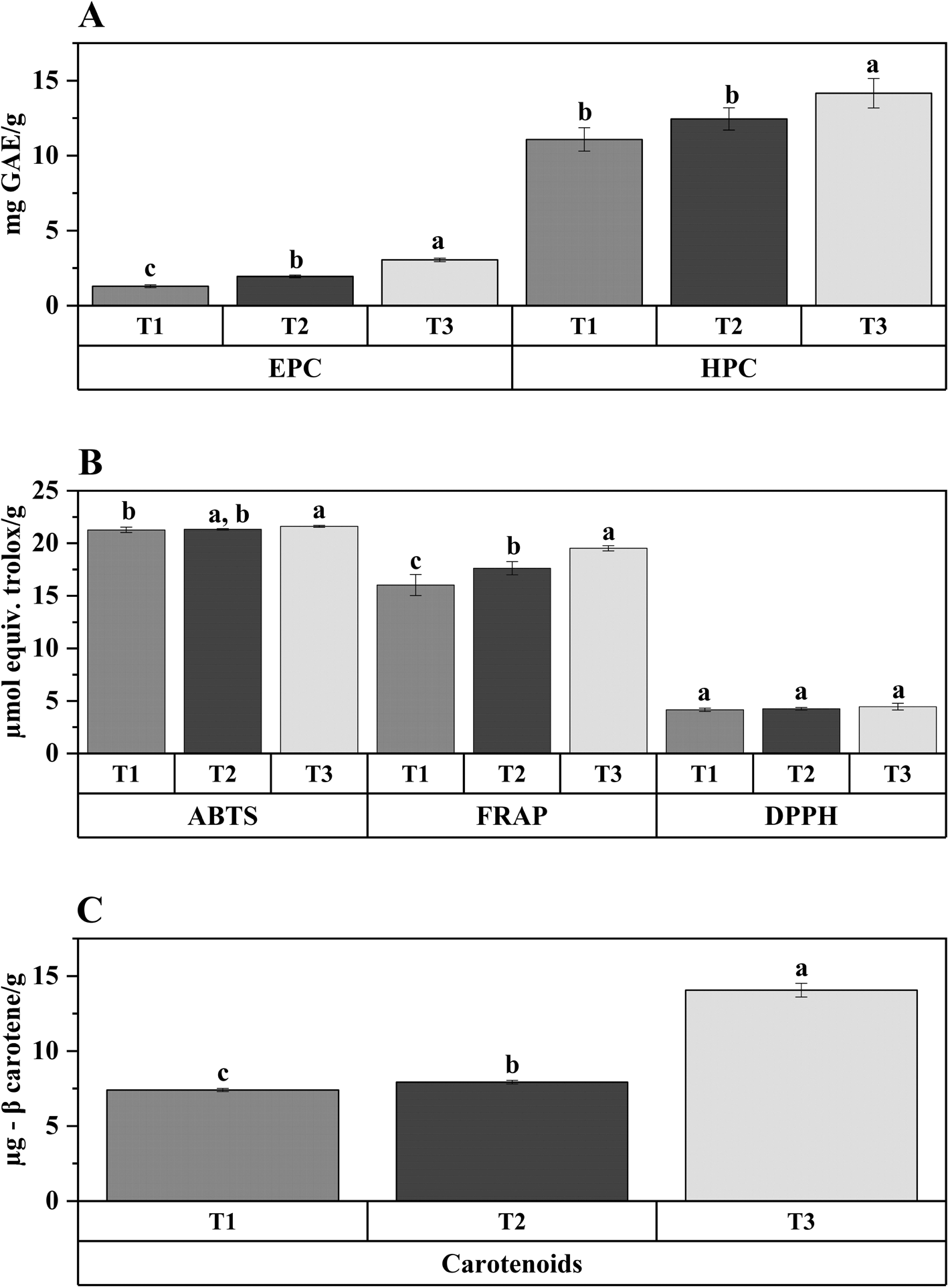
A: extractable phenolic compounds (EPC) and hydroxidizable phenolic compounds (HPC). Values are ± standard deviation. a, b, c: different letters are significantly different (p < 0.05) between treatment.
Antioxidant properties
As seen in Figure 3B, the inclusion of HPQF has an effect on the measured values through ABTS and FRAP (p < 0.05). There was no significant effect with the DPPH method; however, with all the methods, the inclusion of quinoa led to an increase in antioxidant activity.
The extrusion process, which combines high temperatures above 100°C and high pressures, can change the proportion of various phenolic compounds that enhance antioxidant activity. Vanillin and vanillic acid can be produced by thermal decomposition of ferulic acid, while p-hydroxybenzaldehyde can be formed from p-coumaric acid. The caffeic acid present in quinoa is heat-sensitive and may be reduced during thermal processes.45 Some phenolic compounds accumulate in the cellular vacuoles in cereal-based matrices, and it has been shown that the extrusion process can help release phenolic acids by breaking down cellular components and cell walls.10,46
As seen in Table 3, the BI of T3 is the highest (56.29). According to research, the formation of Maillard browning pigments (particularly melanoidins) during the thermal processing of foods has antioxidant activity and can contribute to an increase in polyphenols and the capacity for free radical scavenging. This could be due to the dissociation of the conjugated phenolic fraction during the thermal treatment, followed by polymerization and/or oxidation reactions, and the formation of phenolic compounds that are not present in any of the extruded products (rice/quinoa/sacha inchi).6,47,48 For example,49 developed cookies from quinoa flour, and at a cooking temperature above 180°C, they demonstrated increased stability of the molecules and an increase in antioxidant activity, attributed to the possible decomposition of phenols or their degradation products that could react with the DPPH reagent.50 made cookies from barley and wheat and also attributed the possible increase in antioxidant activity to the formation of proline-L-lysine during the thermal treatments.
The differences in antioxidant content measured through the three methods are due to the nature of each technique. The FRAP assay, in general, is a non-radical-based method based on electron transfer and has little relation to the process of radical scavenging that occurs in lipid systems. It also has little correlation with other measurements of antioxidant activity. On the other hand, the ABTS* assay has been used to measure the total antioxidant activity of pure substances, body fluids, and plant materials. The similarity between FRAP and ABTS may be due to the fact that the latter is also generally classified as an electron transfer-based method that applies the hydrogen atom transfer mechanism. However, the quantification through DPPH does not mimic the radical scavenging mechanism of antioxidants in real foods or biological systems due to the lack of oxygen radicals in the assay.22
The β-carotene content in the snacks significantly increased (p < 0.05) with the addition of quinoa and was found to be in the range of 7.31, 7.93, and 14.05 μg/g db (Figure 3C). The higher carotenoid content in T3 could be attributed to the incorporation of HPQF. Studies have revealed the presence mainly of α-tocopherol and β-tocopherol, 30.18 and 36.11 μg/g, respectively, in whole quinoa flour.51 According to these contents in raw quinoa, it is possible to observe that the extrusion process decreases the presence of carotenoids. Previous studies have shown that the carotenoid content of quinoa extrudates significantly decreases after extrusion and baking, possibly due to the thermal decomposition of these compounds, which are more prone to degradation due to their chemical structure with unsaturated covalent bonds.6 On the other hand, the addition of SI (ingredient) apparently does not contribute carotenoids.52 reported that the total carotenoids in different SI cultivars ranged from 0.07–0.09 mg/100 g of seed, which means that the addition of SI does not increase the β-carotene content in the snacks.
Table 3 shows the textural properties of the extruded snacks, and it can be observed that the inclusion of quinoa has an effect on each parameter (p < 0.05). T3 presents higher values in hardness, indicating that more force is required to deform the food, resulting in lower Nsr values associated with the number of peaks and higher Wcr (crunchiness work). This could be attributed to the higher protein and fiber content and lower starch content (associated with carbohydrates) in T3. According to Ref. 53, the inclusion of protein above 20% has a positive correlation with hardness, which increases due to the reduced expansion caused by protein during extrusion, preventing the formation of air bubbles and resulting in thicker cell walls and therefore harder extrudates.54 developed snacks from soybean residues and also found that higher protein and fiber content, with lower starch content, result in harder and less crispy snacks.
The color parameters found in the snacks were L* values of 68.89, 64.95, and 61.73 for T1, T2, and T3, respectively; a* values of 6.87, 8.34, and 8.78; b* values of 22.66, 23.49, and 23.02; C* values of 5.43, 5.64, and 5.64; h° values of 1.28, 1.23, and 1.21; color difference ranging from 30.98 to 67.08; whiteness index of 60.89, 56.96, and 54.47; and browning index of 46.56, 53.52, and 56.29 (Table 3). Only the b* parameter was not significantly affected by the inclusion of quinoa (p > 0.05).
The increase of HPQF (20, 30, and 50 g/100 g) in the extrudates caused a decrease in L* and WI* as seen in Figure 4, as well as an increase in a* and BI. The highest values of L*, h°, and BI were found in the product with 20% quinoa. The nature of the raw materials used was the main cause of the color changes in the obtained snacks, and this effect was mainly attributed to HPQF, as SIF remained constant in the formulation. This result was due to the effect of quinoa flour inclusion, which caused darkening of the color (browning index) of the mixture, probably due to the presence of Maillard reaction compounds. The color of the extruded products is the result of non-enzymatic browning reactions, and the degradation of pigments present in the raw materials. The caramel color produced during extrusion provides a brown color.
(T1) treatment 1 with addition of 20% quinoa; (T2) treatment 2 with addition of 30% quinoa; (T3) treatment 3 with addition of 50% quinoa.
The lowest a* parameter was found in the snacks with 20% quinoa flour (Table 3), exhibiting a weaker red tone than the other samples. The increase in a* with the increase of HPQF enhanced the red tone of the snacks. The b* parameter of all extrudates leaned towards yellow, and the most intense yellow tone was found in treatment 3. The increase of quinoa flour in the formulation decreased C* and h° parameters, thereby changing the color from yellow to red with the increase of HPQF flour. This browning effect is consistent with what has been reported by other researchers in extruded snacks using insects,55 and it is also in line with the findings reported by Ref. 56 in snacks made from wheat.
The inclusion of 50% quinoa flour significantly increased the protein content, EPC (extractable phenolic compounds), HPC (hydrolyzed phenolic content), antioxidant properties, and carotenoid content. However, texture properties such as Nsr (spatial frequency of ruptures) decreased, and Wcr (crispness work) increased with the inclusion of quinoa, resulting in harder and less crunchy snacks. The color was also significantly affected by the inclusion of HPQF. Snacks with 30% and 50% quinoa content exhibited a darker color, primarily due to the generation of pigments, mainly melanoidins, during the Maillard reaction. This reaction is common during the extrusion process, where carbohydrates and proteins are present.
The possible generation of products during the Maillard reaction could also contribute to the increased antioxidant content measured through ABTS and FRAP assays. However, the DPPH method did not show significant differences.
The inclusion of 25% sacha inchi cake contributed to an increase in protein content and the content of polyphenols and antioxidant compounds in the snacks.
The development of this new gluten-free, high-fiber, and high-protein snack can be considered as a bioactive compound-rich appetizer that meets the demand for nutritious snacks among consumers. At the same time, it would be a good way to increase the consumption of ancestral grains and healthy foods for individuals with gluten sensitivity.
Zenodo: Artículo_sacha_v1. https://doi.org/10.5281/zenodo.8048635. 29
• This project contains the following underlying data:
• FTIR - HyperProteic Quinoa Flour (HPQF) R1: in this file you can find the FT-IR data of the “x” and “y” coordinates of the HyperProteic Quinoa Flour sample of replicate 1.
• FTIR - HyperProteic Quinoa Flour (HPQF) R2: in this file you can find the FT-IR data of the “x” and “y” coordinates of the HyperProteic Quinoa Flour sample of replicate 2.
• FTIR - HyperProteic Quinoa Flour (HPQF) R3: in this file you can find the FT-IR data of the “x” and “y” coordinates of the HyperProteic Quinoa Flour sample of replicate 3.
• FTIR - HyperProteic Quinoa Flour (HPQF) R4: in this file you can find the FT-IR data of the “x” and “y” coordinates of the HyperProteic Quinoa Flour sample of replicate 4.
• FTIR - Sacha Inchi Flour (SIF) R1: this file contains the FT-IR data of the “x” and “y” coordinates of the sacha inchi flour sample of replicate 1.
• FTIR - Sacha Inchi Flour (SIF) R2: this file contains the FT-IR data of the “x” and “y” coordinates of the sacha inchi flour sample of replicate 2.
• FTIR - Sacha Inchi Flour (SIF) R3: this file contains the FT-IR data of the “x” and “y” coordinates of the sacha inchi flour sample of replicate 3.
• FTIR - Sacha Inchi Flour (SIF) R4: this file contains the FT-IR data of the “x” and “y” coordinates of the sacha inchi flour sample of replicate 4.
• FTIR - T1R1: this file contains the FT-IR data of the “x” and “y” coordinates of the T1 sample of replicate 1.
• FTIR - T1R2: this file contains the FT-IR data of the “x” and “y” coordinates of the T1 sample of replicate 2.
• FTIR - T1R3: this file contains the FT-IR data of the “x” and “y” coordinates of the T1 sample of replicate 3.
• FTIR - T1R4: this file contains the FT-IR data of the “x” and “y” coordinates of the T1 sample of replicate 4.
• FTIR - T2R1: this file contains the FT-IR data of the “x” and “y” coordinates of the T2 sample of replicate 1.
• FTIR - T2R2: this file contains the FT-IR data of the “x” and “y” coordinates of the T2 sample of replicate 2.
• FTIR - T2R3: this file contains the FT-IR data of the “x” and “y” coordinates of the T2 sample of replicate 3.
• FTIR - T2R4: this file contains the FT-IR data of the “x” and “y” coordinates of the T2 sample of replicate 4.
• FTIR - T3R1: this file contains the FT-IR data of the “x” and “y” coordinates of the T3 sample of replicate 1.
• FTIR - T3R2: this file contains the FT-IR data of the “x” and “y” coordinates of the T3 sample of replicate 2.
• FTIR - T3R3: this file contains the FT-IR data of the “x” and “y” coordinates of the T3 sample of replicate 3.
• FTIR - T3R4: this file contains the FT-IR data of the “x” and “y” coordinates of the T3 sample of replicate 4.
• FTIR - Whole Rice Flour (WRF) R1: this file contains the FT-IR data of the “x” and “y” coordinates of the Whole Rice Flour sample of replicate 1.
• FTIR - Whole Rice Flour (WRF) R2: this file contains the FT-IR data of the “x” and “y” coordinates of the Whole Rice Flour sample of replicate 2.
• FTIR - Whole Rice Flour (WRF) R3: this file contains the FT-IR data of the “x” and “y” coordinates of the Whole Rice Flour sample of replicate 3.
• FTIR - Whole Rice Flour (WRF) R4: this file contains the FT-IR data of the “x” and “y” coordinates of the Whole Rice Flour sample of replicate 4.
• ITEM ABTS_1: in this file you can see information about the data of antioxidant properties measured by the ABTS method.
• ITEM CAROTENOIDS_1: in this file you can see information about the carotenoid content data present in the snack samples.
• ITEM DPPH_1: in this file you can see information about the data of antioxidant properties measured by the DDPH method.
• ITEM FRAP_1: in this file you can see information about the data of antioxidant properties measured by the FRAP method.
• ITEM Phenolic Compound (EPC y HPC)_1: this file contains information on the polyphenol content data present in the snack samples.
• PARAMENTERS COLOR: in this file you will find the coordinates of the color parameters of the samples.
• TEXTURE T1: this file contains the texture parameter data for the T1 treatment.
• TEXTURE T2: this file contains the texture parameter data for the T2 treatment.
• TEXTURE T3: this file contains the texture parameter data for the T3 treatment.
• TEXTURE T1: this file contains the texture parameter data for the T4 treatment.
Zenodo: Article_sacha. https://doi.org/10.5281/zenodo.7625719. 23
This project contains the following extended data:
• Bacillus cereus-the protocol for the determination of Bacillus cereus present in food according to the colombian regulations is presented.docx
• Coliforms-the protocol for the determination of coliforms present in food according to the colombian regulations is presented.docx
• Fungi and yeasts-the protocol for the determination of fungi and yest present in food according to the colombian regulations is presented.docx
• Mesophiles the protocol for the determination of mesophiles present in food according to the colombian regulations is presented.docx
• Protocol Fiber - The protocol for fiber determination is presented.docx
• Protocol Lipid - The protocol for lipds determination is presented.docx
• Protocol Protein - The protocol for protein determination is presented.docx
• Salmonella Spp - The protocol for the determination of Samonella present in food according to the colombian regulations is presented.docx
• Staphylococcus aureus - The protocol for the determination of Salmonella present in food according to the colombian regulations is presented.docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Aug 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)