Keywords
Meta-Analysis, Diabetes Gastroparesis, Diabetes Mellitus, Ultrasonography
Meta-Analysis, Diabetes Gastroparesis, Diabetes Mellitus, Ultrasonography
Gastric symptoms of diabetes are often a long-standing complication of uncontrolled diabetes mellitus (DM) which highly affect the patient's quality of life.1,2 Delayed gastric emptying (DGE) without obstruction in diabetes gastroparesis (DG) is caused by disorder in the neuromuscular function of the stomach which can be a result of various processes: idiopathic, DM-associated gastroparesis, or as an adverse effect of medical intervention such as surgical gastric resection.3,4 Diabetic gastroparesis is shown by gastrointestinal symptoms such as nausea, vomiting, postprandial fullness, bloating, and early satiety.4,5 Hyperglycemia, oxidative stress, neuropathy, and inflammation in diabetic patients are associated with the pathophysiology of DG.3,5
A study in general practice from the United Kingdom revealed that the prevalence of gastroparesis per 100,000 persons was 13.8 in 2016. DM is the second most common etiology after idiopathic.6 Different from that record, a study in the United States in 2018 found DM as the primary cause of gastroparesis, with as high as 57.4% of the patients having DM. The overall number is also higher, reaching 267.7 per 100,000 persons, although the prevalence of patients with definite delayed emptying reported from scintigraphy is lower, with only 21.5 per 100,000 persons.7 It is estimated that the prevalence of this disorder to be 9.6 per 100,000 person in men and 37.8 per 100.000 in women.8 Depression and anxiety are common in patients with gastroparesis; thus, their quality of life is significantly impaired.5,9 Aside from the quality of life, evaluation of delayed emptying is also essential to analyze absorption and consequently regulation of blood glucose in DM.1,5
The gold standard for diagnosis of DG is with gastric emptying scintigraphy (GES) examination.4 However, the application of this method is still limited in many countries due to the limited availability of examination equipment, relatively expensive cost, and long hours of the examination.10,11 Radiation exposure is also a concern in itself.12 Other recommended alternatives such as 13C-octanoic acid breath test, wireless motility capsules, and electrogastrography (EGG) are even less available. In contrast, ultrasonography (USG) is readily available in most hospitals worldwide. USG examination is a non-invasive, affordable, and relatively easy-to-perform test. It can measure emptying time, intra-gastric volume, and antral cross-sectional area (CSA).8 It is also known to be used by anesthesiologists to determine the volume and content of the stomach for perioperative point-of-care tests.13–15 The broad availability, safety from radiation exposure, and affordable tools could make ultrasound a suitable candidate for an alternative gastric emptying examination16–18 and its accuracy compared to the gold standard needs further investigation. The potential USG use on DG screening remains a question. The objective of this study is to1 evaluate USG findings of gastric changes found in DM and DG. 2
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-2020 guideline.19,64 Prior to this publication, the study protocol had already been registered to the PROSPERO database (CRD42022328695 (10/05/2022)).20
Literature search was conducted during period April 30 – May 6, 2022 by comprehensive search in Google scholar, PubMed, Science Direct, ProQuest, and Scopus by authors. The search words for each database platform were based on Medical Subject Heading (MeSH) terms combined with Boolean operator (AND/OR) with keyword: (“gastroparesis”[MeSH Terms] OR “gastroparesis”[All Fields]) AND (“diabetes mellitus”[MeSH Terms] OR (“diabetes”[All Fields] AND “mellitus”[All Fields]) OR “diabetes mellitus”[All Fields] OR “diabetes”[All Fields]) AND (“diagnostic imaging”[Subheading] OR (“diagnostic”[All Fields] AND “imaging”[All Fields]) OR “diagnostic imaging”[All Fields] OR “ultrasonography”[All Fields] OR “ultrasonography”[MeSH Terms]) (PUBMED), (Diabetic Gastroparesis AND Ultrasonography OR USG OR US) in Google Scholar, Science Direct, ProQuest, Scopus, and Diabetes Gastroparesis in clinical trial registries (i.e clinicaltrial.gov, ISRCTN, European Clinical Trial Registry) (see Extended data for the full list of clinical trial registries64).
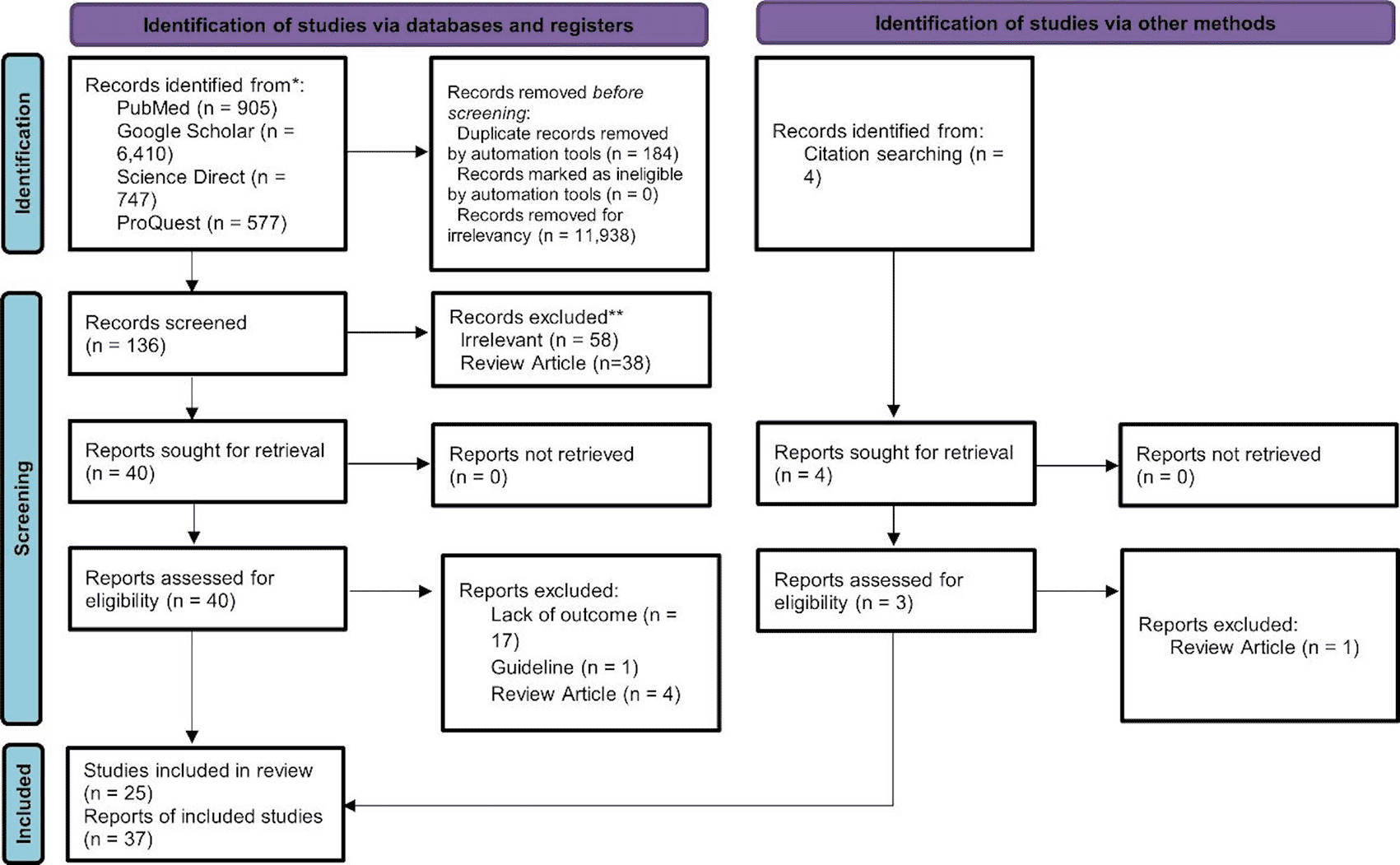
The studies gathered from the search were screened by the authors. Duplicates were removed using Mendeley Reference Manager after a manual crosscheck. Reviewers independently screened the studies based on the eligibility criteria: observational (cross-sectional, case-control, and cohort) and interventional studies, included patients with both Type 1 (T1D) and Type 2 Diabetes (T2D), assessed comparison of gastric antral area on fasting and postprandial state, gastric emptying time, gastric half emptying time, antral contraction rate. Exclusion criteria were: review articles, duplicates, protocols or guidelines, irrelevant articles, case series, case reports, and non-human studies. The study selection process from searching, screening, and selection is elaborated further in the PRISMA diagram (Figure 1).
Reviewers extracted the data separately with a standardized format and reviewed. Any differences and disagreements were resolved through discussion among authors until an agreement was reached. Standardized formats for data extraction were as follows: author name, publication year, country, study design, sample size, age, duration of diabetes, examination methods, outcomes, and quality assessment.
All authors performed quality assessment. The study was assessed with the Study Quality Assessment Tool from the National Institute of Health (NIH) for case-control, cohort, and cross-sectional studies.21 Quality assessments for cross-over studies were assessed based on Cochrane Systematic Reviews.22 The outcome of the quality assessment was assigned as poor, fair, and good based on the overall rating from each question. Differences were resolved through discussion among authors until an agreement was reached. This article included all studies from all ranges of quality (poor, moderate, and good). Quality assessments are shown in Table 1.
The calculations for each operational definition were as follows. Cross-sectional area is defined as total gastric cross-sectional area evaluated during ultrasonography assessment with multiple method (i.e. anteroposterior diameter × craniocaudal diameter × π)/423). Preprandial/fasting antral is defined as the measurement of antral area pre-meal or after fasting time, while postprandial is measured post-meal.24 Gastric emptying time is described as the time after the meal until the time gastric returns to its original measurement.25 Gastric half-emptying time (GE50) is the time post-meal until only 50% of gastric content is left.26 The motility index is calculated by mean amplitude × frequency of contraction.27
The primary outcomes of this study were to analyze the effect of gastric changes in diabetic and DG patients found in ultrasound examination. This study's gastric changes consisted of antral area CSA, GET, GE50, GER (%), and antral contraction. Dersimonian-Laird method was used for tau estimation. Inverse variance was used for pooling of SMD of continuous outcome. P-value of <0.05 is the cutoff for study to be considered statistically significant.
I2 and Q statistics were used to assess heterogeneity. High heterogeneity was defined as I>50% and/or P-value <0.1. Random-effect analysis was used for estimating the effect size regardless of the I2 value. Leave-one-out sensitivity analysis was conducted to evaluate each study's influence on the pooled results. Potential bias in publications was assessed via Begg's funnel plot. This study used a subgroup analysis based on group population in the study design. Sensitivity analysis was used to ensure the robustness of the result. All statistical analyses were performed using Review Manager ver. 5.4 (The Cochrane Collaboration).
The search strategy described in Figure 1 resulted in 136 articles from database screening, four studies from manual screening, and three from citation screening. After evaluation of titles and abstracts, 43 articles were selected for full-text evaluation. The review yielded 25 case-control studies. Article appraisal graded 18 articles as good, four as fair, and two as poor (Extended data¸supplemenent 164). All studies were included in the study to minimize the risk of publication bias.
All studies reported gastric changes found in people with diabetes with various degrees. The gastric antral area, emptying time, and antral contractility were found to be worse in DM and DG populations. Changes in antral contraction were found to be insignificant in diabetes but less in the DG population. With a total of 25 studies included, 715 samples of DM were included in this study. Further result are elaborated in Table 2.
Summary meta-analysis results presented as forest plots are shown in Table 3.
Antral cross-sectional area
The standardized mean difference (SMD) of antral CSA in pre- and post-prandial showed that antral CSA (cm2) in diabetic patients were significantly bigger both in preprandial (SMD=1.25 cm2 (95%CI, 0.79 cm2 to 1.71 cm2), I2=81%, P<0.05) and postprandial (SMD=3.70 cm2 (95%CI, 1.45 cm2 to 5.34 cm2), I2=85%, P<0.05). Leave-one-out sensitivity analysis in the preprandial subgroup showed that heterogeneity was reduced when the study by Darwiche et al., 2014 was omitted from the analysis (I2=70%, P<0.05) with the direction of effect remaining unchanged. In the postprandial subgroup, heterogeneity was reduced following the removal of studies by Darwiche et al., 2014 (SMD=1.06 cm2 (95%CI, 0.7 cm2 to 1.42 cm2), I2=65%, P<0.05) and Taher et al., 2016 (SMD=1.26 cm2 (95%CI, 0.71 cm2 to 1.8 cm2), I2=76%, P<0.05), however, the direction of effect remains Figure 2.
Gastric emptying time (GET)
The SMD of gastric emptying time (GET) in DM and DG revealed that GET (min) were significantly longer in both the DM and DG subgroups. Furthermore, a longer emptying time in DG (SMD=75.44 min (95%CI, 31.61 min to 119.27 min), I2=97%) was found compared to DM (SMD=35.54 min (95%CI, 11.49 min to 59.60 min), I2=94%). Leave-one-out sensitivity analysis in both subgroups showed no significant reduction in heterogeneity if each study was removed Figure 3.
Gastric half emptying time (GE50)
The SMD of GE50 in DM showed that GE50 were significantly longer in both DM and DG subgroups. A bigger time difference to HC was found in DM (SMD=20.73 min (95%CI, 14.69 min to 26.77 min), I2=60%, P<0.05) compared to DG (SMD=7.46 min (95%CI, 5.06 min to 9.86 min), I2=68.2%, P<0.05). Leave-one-out sensitivity analysis in the DM subgroup showed that heterogeneity was reduced (I2=44%, P<0.05) when the study by Brogna et al., 1990 (SMD=18.34 min (95%CI, 11.73 min to 24.94 min)) was removed from the analysis. No changes of direction were found in leave-one-out sensitivity analysis Figure 4.
Gastric emptying rate (GER)
The pooled result of SMD of gastric emptying rate (GER) in DM and DG were smaller in both subgroups compared to the control. Overall, DG subgroups had smaller GER (SMD=-31.95% (95%CI, -42.22% to -21.69%), I2=84%, P < 0.05) than DM (SMD=-16.14% (95%CI, -30.88% - -1.39%), I2=78%, P<0.05). Leave-one-out sensitivity analysis in the DM subgroup revealed that heterogeneity was reduced when the study by Darwiche et al., 1999 was removed (I2=0%, P=0.9). In the DG subgroup, sensitivity analysis showed no significant reduction in heterogeneity if each study was removed. No changes of direction were found in leave-one-out sensitivity analysis Figure 5.
Antral contraction
The frequency of antral contraction in diabetic patients were found similar to HC (SMD=0.09 (95%CI, -0.18 to 0.36), I2=0%, P=0.49), but significantly less in DG (SMD=-1.47 (95%CI, -2.61 to -0.33), I2=82%, P<0.05). Leave-one-out sensitivity showed that heterogeneity reduced when the study by Kawagishi et al., 1994 (SMD=-1.97 (95%CI, -2.93 to -1.00), I=42%, P=0.16) or Sogabe et al., 2005 (SMD=-1.12 (95%CI, -2.18 to -0.07) I2=64%, P ≤ 0.05) was removed from the analysis. The direction of effect remains unchanged in both treatments Figure 6.
Begg’s funnel plot inspection for asymmetry to assess the publication bias in this study indicate possible publication bias in outcomes of antral area and GE50, the rest of the outcomes (GET, GER, and antral contraction) were found symmetrical, hence minimal risk of publication bias were found in the variables Figure 7.
Diabetes with and without gastroparesis results in changes in gastric physiological conditions that can be examined via ultrasonography. In our study, 11 case-controls demonstrated changes in gastric antral CSA,17,23–26,28–33 indicating there is a possible structural change leading to a wider antral area (SMD 1.25 cm2 in fasting state and 3.70 cm2 in postprandial) compared to the HC. Total GET16,18,30,34–37 and GE5012,26,34,38,39 were also assessed, and all studies reported longer emptying time in DM and DG groups (SMD to healthy control of GET in DG at 75.44-min, DM st 35.54-min; GE50 15.75-min in DM and 7.46-min in DG; and reduced GER SMD -31.95% in DG and -16.14% in DM). Reduced gastric motility17,27,40–42 was also found in DG (SMD to control at -1.47 contraction).
Antral area and antral dilation were found to be wider in both fasting and postprandial states than HC in our study. Undeland et al. (1996)31 reported the loss of parasympathetic innervation presented by the loss of vagal tone and the finding of wider fasting and postprandial antral area. Further study by the same author33 reported that loss of vagal tone is associated with the antral area and intragastric meal distribution. The mechanism might be related to vagal nerve function to stimulate gastric acid secretion to prepare the stomach for digestion. In DM and DG, acid secretion was found to be lower43 with slower emptying of gastric juice,29 which may be resulted from vagal neuropathy.31,33 Autonomic neuropathy due to vagal impairment also causes loss of gastric tone32; consequently, wider dilation before and after the food filling can be seen in DM.
Prolonged emptying time in DM is often associated with two mechanisms; hyperglycemic condition and the presence of autonomic neuropathy.5 The first mechanism is a hyperglycemic condition. Moldovan et al. (2005)30 discovered that HbA1c level and fasting glucose are highly significant with DGE. Maheshwari et al. (2021)44 stated the prevalence of DGE to be around 30–50% in DM patients. In acute hyperglycemia, disturbance of the physiologic gastric emptying phase is associated with prolonged gastric emptying. Gastric emptying of solids is divided into two phases; the lag phase (movement of meals from the fundus to antrum) and the post-lag phase (the process of meal passing the pylorus), which involves actions of the pyloric, antral, and proximal gastric section. Conditions of hyperglycemia can stimulate pyloric contraction, disrupt antral wave, and reduce proximal gastric tone, causing a longer lag phase and slower post-lag emptying rate.45 Chronic hyperglycemia can also cause prolonged gastric emptying. In the normal state, blood glucose and incretin secretion depend on carbohydrate exposure in the small intestines. However, constant hyperglycemia in poorly controlled DM can induce glucagon like peptide-1 (GLP1) and gastric inhibitory polypeptide (GIP) production, which stimulate insulin production, resulting in a constantly high level of GLP1.46 GLP1 slows gastric emptying rate.47,48 Furthermore, the hormonal by-product from incretin secretion, amylin, induces vagal response and further decreases the gastric emptying rate in both DM and DG population.49 Therefore, both acute and chronic conditions may lead to prolonged gastric emptying in DM.
The second mechanism that often-prolonged gastric emptying shown in DM is neuropathy.5 The gastric area receives innervation from celiac ganglia and vagal nerve, which regulate gastric motility.50 Diabetes causes neuropathy that reduces antral contraction, causing less contraction and resulting in slower food digestion in the stomach and, eventually, longer emptying time.5 A study by Kawagishi et al.40 and Vogelberg et al.38,41 finds that autonomic neuropathy is associated with reduced antral contraction. The prevalence of neuropathy in DM with DGE from the studies varies, ranging from 39%–75%, indicating a possible correlation to DGE. Seven studies25,30,33,40,51,52 found a significant relation in DGE to autonomic neuropathy (AN) in DM. However, Pfaffenbach et al. (1994)36 and Zhou et al. (2019)53 found no significant difference in DGE in DM with AN or without AN. These findings are congruent to the theory of prolonged GET due to neuropathy.
Gastric half emptying time in diabetic patients showed an interesting result. The finding showed SMD to HC of 15.75-min in DM and 7.46-min in DG, with longer GE50 in DM, contrary to the definition of DG itself, which is an established diagnosis after findings of definite prolonged gastric emptying with the absence of structural obstruction.54 The longer GE50 in DM is because studies included in the meta-analysis in DG used a liquid test-meal,12,39 while all samples in DM were measured with a solid test-meal, which requires a longer time to digest.26,34,38 Therefore, a slower half-emptying time was found in the solid meal test in DM compared to liquid test in DG populations.
The method for ultrasonography assessment of gastric emptying varies across all studies. To the best of our knowledge, a protocol for gastric emptying measurement has not yet been established, especially in DG. The fasting time varies for every study, with the shortest overnight fasting (6~8 hours)31,33 and the longest at 16 hours.34 The meal sets to evaluate postprandial gastric changes in both liquid and solid meal test were also very diverse. Various liquid meals were used in the liquid meal test, including water,51 consommé,27,42 meat soup,1,26,31,32 soluble dietary fiber liquid,55 and commercial meal soup.1,33 For meal test, a variation on solid meal test includes World Instant Gastrointestinal Ultrasound Contrast,12,56 test meal with different calorie content (280–650kcal),30,35,36,38,41,57 rice pudding,15,28,29,52,58 minced beef meat,10 gastro-enteric ultrasound developer meal,37 and semi-solid paste.59 An established protocol for meal-set and fasting time is necessary to reduce the risk of bias in the gastric emptying study.
Routine use of ultrasonography for gastric emptying has multiple benefits. A study by Stevens et al. (2011)10 analyzed the difference in gastric emptying measurement using 2D-USG compared to the 3D-USG method and found no significant correlation in results from both examination methods. However, 3D-USG was found to be superior due to its significance and relatively good agreement limit when compared to the previous gold standard GES.60 Two studies28,36 analyzed the inter-observer and intra-observer variability in USG to assess the reproducibility of USG and found the inter-observer standard measurement of error (SME) around 0.3%–10.9% and intra-observer SME around 3.6%–9.5%, indicating a good reproducibility for USG. GES and USG comparison shows a good correlation with a limit of agreement within 2SD15 and no significant difference on result.10,12 Bian et al. (2011)25 compared the octane breath test to USG and found a significantly similar result on variables. Another study by Jūngling et al. (2001)51 used USG as the standard method to assess oatmeal test sensitivity and specificity. All results showed a favorable tendency on USG use for gastric emptying measurement except for one study by Maheshwari et al. (2021),44 which found poor concordance in gastric evaluation compared to endoscopy to examine residual gastric volume. However, the poor correlation needs further investigation as it might be resulted from other factors (i.e., poor measurement technique, suboptimal positioning, clinician's expertise, etc.).44 The capability of USG to assess real-time emptying and the gastric area remains superior for USG compared to GES.28,29 Furthermore, Zhou et al. (2001),61 Murray (2005),57 and Vogelberg (1988)17 also highlight the possible use of USG to monitor gastric improvement after ghrelin, metoclopramide, and cisapride medication. Therefore, the use of USG in GES requires a standardized clinical trial or more extensive study to evaluate the extent of function and possible use of USG in gastric emptying assessment.
Abdominal symptoms were mostly complained by the patient with DM and DG with DGE. Chiu et al. (2014)26 reported that GI symptoms score in DM postprandial decrease less than HC. The top three most complained symptoms were abdominal distension (36.1%–54.7% prevalence),23,26,30 postprandial fullness (19.4%–54.7%),23,30 and dysphagia (38.9%).30 The food process in the stomach is complex and requires coordinated actions of all stomach regions. Starting from reservoir function of the fundus, peristaltic mix in the antrum, and lastly, gastric pylorus as the gate filters before the chyme enters duodenum.2 Other structures were also involved in the food process, including smooth muscles, vascular system, enteric and extrinsic autonomic nerve, and the interstitial cells of Cajal.62 In diabetic patients, changes in gastric anatomy,25,26 supporting structures51,56,62 (i.e., autonomic innervation, vascular system),62 impaired gastric content distribution,33 immunological changes,63 and systemic conditions (i.e., hyperglycemic state, hormone production)40 were found. These changes disrupt the physiological process of gastric digestion, leading to alteration of emptying time to the complaint of gastric symptoms.62 Delays in gastric emptying in DM itself is a spectrum of gastric change, leading to gastroparesis.
There are some limitations in our study. We found that there are very limited data for ultrasonography measurement in a certain population (i.e., patient with marked gas retention, markedly obese due to inability to assess USG results objectively); some studies excluded obese27,42 patients for better representation of the general population. Therefore, further evaluation of ultrasonography measurement in this population is needed. We included postprandial data for every various meal set for each study. A standardized protocol in solid or liquid food packages to evaluate gastric emptying must be established to ensure a correct assessment. Long examination hours for USG (range from 30 minute38 – 8 hours53) might not be viable for every situation. Therefore a further study to evaluate shorter analysis, or a standardized time for USG assessment is advised. We also acknowledge that there are potential publication bias in our study based on the Begg’s funnel plot assessment. Aside from the limitations, to the best of our knowledge, this is the first meta-analysis that evaluates the ultrasonography findings in DM and DG that includes various study designs with no language exclusion and included studies from all countries worldwide.
In conclusion, DM and DG could affect gastric anatomic and physiologic function, which results in prolonged emptying time and gastric symptoms. Wider antral area, longer gastric emptying time, longer half-emptying time, reduced gastric emptying rate, and less antral contraction were found in both populations. This study supports the use of ultrasonography for screening and diagnostic methods to treatment monitoring for DGE. However, these findings were based on observational studies, in which nonuniform definitions exist for several outcomes. Therefore, randomized controlled trials are advised for future studies.
All data underlying the results are available as part of the article and no additional source data are required
Open Science Framework: Ultrasonography Findings in Diabetes and Diabetes Gastroparesis: A meta-analysis. https://doi.org/10.17605/OSF.IO/YQ4XN. 64
This project contains the following extended data:
- Suppl 1 Literature Search.docx (Literature search methodology, Boolean keywords, and list of databases)
- Suppl 2 Quality Assessment.xlsx (Quality assessment of the included studies)
- Suppl 3 Forest Plot for all outcomes.docx (Meta-analysis forest plots for all outcomes)
- Suppl 4 Begg’s Funnel Plot for all outcome.docx (Begg’s funnel plot assessment for evaluation of risk of bias of the included studies)
- Database search.xlsx (Raw datavase search result)
Open Science Framework: PRISMA checklist for ‘Ultrasonography findings in diabetes and diabetes gastroparesis: A meta-analysis’. https://doi.org/10.17605/OSF.IO/YQ4XN. 64
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors would like to thank the division of Gastroenterology, Department of Internal Medicine, Central General Hospital Cipto Mangunkusumo and Staffs of Faculty of Medicine, Universitas Indonesia for the support throughout the writing process. We thank our colleagues, Humaira Jasmeen and Livia Assyifa Rachman who provided insight and useful suggestions.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: gastroenterology; motility
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 31 Aug 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)