Keywords
Arsenic exposure, uterus inflammatory, vulva absorption, vulva exposure
This article is included in the Global Public Health gateway.
Arsenic exposure, uterus inflammatory, vulva absorption, vulva exposure
Heavy metal exposure is still often found in humans.1 Heavy metals in the environment come not from industrial pollution as well as nature itself.2 Hot springs,3,4 water blowouts,5 volcanic eruptions, and abrasion are examples of the natural activities that produce and supply the heavy metal into environment.6 This makes heavy metal exposure to the organisms difficult to avoid. Therefore, assessing potential pollution, heavy metal exposure mechanisms, and their health effects are important.7 The effects of heavy metal exposure on an organism are significant, since it could easily interact with many organs.8,9 Frequent exposure can also deposit the metal inside the body.10 Although the organism has its own outer protective barrier,11,12 it still has some open organs, which can be the heavy metals’ point of entry into the body.13
The female external genital organs are open14 and they could become the entrance for toxic agents into the body. The tissue structure of the vulva and vagina is more permeable than the skin because vulvar tissue has the ability to hydrate and reduce water barrier function.15 In addition, most of the vaginal wall is covered by blood and lymphatic vessels, and therefore chemical exposure might be directly absorbed into the circulatory system.16 It is also very easy for the vulva and vagina to absorb toxic chemicals, and absorbed chemicals can be easily and effectively distributed through the body.17
The entry of harmful chemicals into the body through the female reproductive organs can be caused by many activities. Most are linked to the use of feminine hygiene or feminine care products.18 These various products are used by attaching them to the vulva, smearing, douching, and evaporating into the vagina or through immersion. The basic ingredients of these products can contain dioxin, chlorine, pesticide residues, methyldibromo glutaronitrile, methylchloroisothiazolinone, methylisothiazolinone, parabens, quaternium-15, DMDM hydantoin, and other hazardous substances.17 Apart from these various products, other activities that allow the entry of harmful chemicals into the body through the genital organs can occur while bathing, wiping, swimming, and soaking.
Women in Aceh Province, Indonesia use river estuaries for their daily livelihoods. They collect various estuarine biota such as oysters and shellfish at the bottom of the estuary by sitting or squatting in the estuary. During work, estuary water containing hazardous substances wet the genital organs. A study found that the work is performed for 2–3 h/day without using personal protective equipment.13 The north zone of the geothermal area around Seulawah Agam volcano of Aceh Province has a hot spring feature.19,20 This hot water flows and joins the nearest river21 such as Ie Seu Um geothermal river that crosses a residential area in Mesjid Raya Sub-District, in the Aceh Besar District.22 This river contains various levels of arsenic: the highest level found in water sources was 5478 μg/L (5 mg/L); the arsenic level in local wells was 800 μg/L (0.6 mg/L), and those at river estuaries was 300 μg/L (0.3 mg/L).23 Meanwhile, the quality standard concentration for arsenic level in water set by the World Health Organization (WHO) and the Ministry of Health of the Republic of Indonesia is only 10 μg/L (0.01 mg/L).24,25
The effects of arsenic on the female reproductive system not only cause toxicity to the reproductive organs, decreases libido, and teratogenic disorders, but it could also affect the well-being of a fetus, for example through congenital malformation.26–28 Arsenic exposure to the body causes metabolic toxicity in cells as well as oxidative stress and as a result, causes lipid peroxidation, membrane damage, membrane peroxidation and destruction, which might lead to cell death.29 Many studies assessed the effects of arsenic exposure through oral, ingestion, inhalation, parenteral, and dermal on damages to the reproductive system. However, research assessing the effect of arsenic exposure through the vulva and vagina on uterus damages is limited.
The present study aimed to assess the effects and damages of arsenic exposure through the vulva on the uterus using animal model. The concentration was adjusted to the arsenic levels in the geothermal area of Ie Seu Um, Aceh Besar Regency (5, 0.8, 0.3, and 0.01 mg/L) and the exposure was applied for 2, 4, 6, and 8 weeks. The oxidative effect was examined through the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and hydrogen peroxide (H2O2) of the uterus tissue. Uterus inflammation was studied by measuring the level of tumor necrosis factor α (TNFα) and examining the histopathology of the uterine tissue.
Female Sprague-Dawley rats (Rattus norvegicus L.) aged 6–24 months and weighing 200–300 g were used in this study. The model animals were purchased from the Abadi Jaya Farm in Yogyakarta, Indonesia. All animals were healthy confirmed with active movements, ate well, hair did not dull or fall out, with bright eyes, and were not blemished. All animals were reared in a controlled temperature environment (25°C±2°C) at a 12-hour dark/light cycle. All efforts were taken to minimize animal suffering during the study: the animal facility was cleaned and disinfected regularly, the animal facility was kept quiet with controlled environmental conditions, and the animals were supplied with c-05 pellets (Citra Ina Feedmill, Jakarta, Indonesia) and water from the national water utility company ad libitum. Before treatment, all rats were acclimatized for seven days.
The animals were divided into five groups, consisting of group K0 (control group), K1, K2, K3 and K4 with arsenic concentration of 5 mg/L, 0.8 mg/L, 0.3 mg/L and 0.01 mg/L, respectively. The control group did not receive any exposure but was placed in the same condition as the treatment groups. Each group (K0-K4) consisted of six rats; during the arsenic exposure process, the animals in each treatment group were placed in separate plastic tub covered with woven iron wire with the same room conditions. All treatment groups were exposed to arsenic over four durations: 2, 4, 6 and 8 weeks (i.e., 14, 28, 42 and 56 consecutive days, respectively).
The study was conducted from September to December 2020 in the Laboratory of Chemistry/Biochemistry, Faculty of Medicine, Universitas Lambung Mangkurat, Banjarmasin, Indonesia. The protocol of the study was reviewed and approved by the Ethics Committee of the Medical Research, Universitas Lambung Mangkurat (No. 259/KEPK-FK UNLAM/EC/VII/2020, dated 30 July 2020).
Arsenic trioxide with a purity of 99%, purchased from Loba Chemie Laboratory Reagents and Fine Chemicals (Loba Chemie Pvt Ltd, Maharashtra, India), was used to make the arsenic solution. The solution was made by dissolving 5 mg, 0.8 mg, 0.3 mg, and 0.01 mg of arsenic in 1 L of water to produce concentrations of 5 mg/L, 0.8 mg/L, 0.3 mg/L and 0.01 mg/L, respectively. These arsenic levels correspond to the arsenic levels in the geothermal river flow of Ie Seu Um, Aceh Besar District, Aceh Province, Indonesia.30
The arsenic exposure was carried out by immersing the vulva of animals. Soaking was done in a 39 × 42 × 15 cm plastic tub covered with woven iron wire. One soaking tub contained one rat; the immersion limit was as high as the tail until the entire vulva was submerged. The arsenic exposure was conducted over 2, 4, 6, and 8 weeks (i.e., each treatment group had four different exposure times). The exposure was conducted 2.5 h/day. This duration was based on the average length of time that female workers forage for oysters in the estuary of the Ie Seu Um geothermal river.13
One day after the end of exposure periods (2, 4, 6 or 8 weeks for K1, K2, K3 and K4, respectively) the animals were euthanized by injecting ketamine 150 mg/kg-xylazine 10 mg/kg intraperitoneally following the guideline of Animal Euthanasia Policy from the Institutional Animal Care and Use Committee.31 The uterus was removed during surgery, cut into small pieces and fixed using a phosphate buffer solution with a pH of 7. The small pieces of uterus were mashed to form 5 mL of liquid and centrifuged for 10 min at 3500 rpm.32 The supernatant fraction was collected and used directly to measure the levels of MDA, SOD, H2O2, and TNFα.
A total of 100 μL of uterus homogenate was mixed with 550 μL of distilled water, 100 μL of 10% trichloroacetic acid (TCA), 250 μL of 1 N HCl, and 100 μL of 1% Na-Thio (EMD Millipore Corporation, Billerica, USA). The mixture was then homogenized and centrifuged for 10 min at 500 rpm. Then, the top layer was collected and heated in a water bath at 100°C for 30 min and then cooled at room temperature (25±2°C). The absorbance was measured with a UV–Vis spectrophotometer at a maximum wavelength of 532 nm.33
A total of 100 μL uterus homogenate to be measured for SOD levels was incubated for 5 min using 3 mL of 0.05 M Na2CO3 and 3 mL of 0.1 M EDTA with a pH of 10.2 and added with 100 μL of adrenaline. The initial absorption was measured (A0) using a spectrophotometer (λ = 480 nm). The solution then incubated again at 30°C for 5 min before determining the final absorption (A1).34
A total of 1 mL of uterus homogenate was added to 5 mL of phosphate buffer and slowly homogenized. Then 1 mL of the product of this reaction was taken and 2 mL of dichromate/glacial acetate was added. Heating was done if blue precipitates were formed by heating the tube in a water bath for 10 min until a green color from chromic acetate was formed. The tube was then cooled to room temperature (25 ± 2°C), and H2O was added to a volume of 3 mL. Next, the solution was placed in a cuvette, and its absorbance was measured by UV–Vis spectrophotometer (λ = 570 nm).35,36
The level of TNFα was measured using the enzyme-linked immunosorbent assay (ELISA) method. The Rat TNFα ELISA kit was used following the manufacture’ protocol (NB-E30635, Novatein Biosciences, Massachusetts, USA). The ELISA was conducted according to the manufacturer’s instructions and the absorbance was read at a wavelength of 450 nm within 30 min.
The uterus tissue was immersed in 10% formalin, cut to a size of 10–15 mm × 10–15 mm × 5 mm, and then proceeded with dehydration, clearing, impregnation, and embedding following standard protocol in Anatomy Pathology.34 Briefly, the tissues were sliced with a thickness of 3–5 μm using a microtome before hematoxylin-eosin (HE) staining. The slices were placed on a slide, immersed in xylol (2 min), absolute ethanol (1 min), and 95% ethanol (1 min) sequentially, then added with water for 10–15 min, and immersed four times in water. The slices were then soaked for 3 min with a hypo solution, rinsed with water for 10 min, then soaked for another 5 min in Mayer’s hematoxylin solution, and watered for approximately 20 min. The preparation was then covered with a coverslip and glued with adhesive.
The uterus histopathology assessment was performed by counting the average number of cells involved in inflammation (macrophages, neutrophils, and lymphocytes). Observations were made using a Meiji Techno microscope with a Sigma HDMI digital photo, 400× magnification. Observation of inflammatory cells was conducted using TopView software through 10 fields of view by paying attention to the histological zone structure of the three preparations found on the slide. The number of inflammatory cells was obtained from the average number of macrophages, neutrophils, and lymphocytes.37 To reduce bias in the study, two pathologists did the observation.
Data were presented as mean ± standard deviation (SD). To compare the levels MDA, SOD, H2O2, TNFα and inflammatory cell counts among groups, ANOVA test was used. Duncan’s multiple range tests were used to compare MDA, SOD, H2O2, TNFα and inflammatory cell counts between exposure times. All analyses were conducted using a Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc., NC, USA).
Among treatment groups, the highest MDA level was found in the K1 group (5 mg/L) exposed for eight weeks (224.33 ± 1.75 μM) while the lowest concentration was seen for K4 group (0.01 mg/L) exposed for two weeks (174.33 ± 0.51 μM) (Table 1). In the control group, the average MDA levels for the four different exposure times was approximately the same (174 μM). Our data indicated that the higher the arsenic concentration and the longer the exposure time, the higher the uterus MDA levels.
The ANOVA test indicated a significant difference in MDA levels in all groups (p < 0.0001) (Table 1). Duncan’s multiple range tests were used to compare the MDA level between exposure times in each concentration group. The significant difference between exposure time is indicated by different superscript letters (Table 1). There were significant differences in MDA levels between exposure times in all arsenic concentration groups, except for the concentration of 0.01 mg/L exposed for 2 weeks, for whichthere was no difference between control and the 0.01 mg/L group.
The SOD levels in the uterus after exposure to arsenic at different concentrations and exposure times are presented in Table 2. The highest SOD level observed in K4 (0.01 mg/L) group exposed for two weeks while the lowest SOD level was in the K1 (5 mg/L) group exposed for eight weeks. The levels of SOD were significantly different between groups (treatment and control group) with p < 0.0001.
Our data suggest that the longer the exposure time, the lower the levels of SOD. There was a decreasing trend with the length of exposure time. In the K1 group for example, exposure to arsenic at a concentration of 5 mg/L for two weeks produced a SOD of 1.47 ± 0.34 U/mL, and this decreased to 0.36 ± 0.08 U/mL after eight weeks. Duncan’s multiple range test in this group found that there were differences in SOD levels at two, four, six, and eight weeks; however, there was no difference between four and six weeks, nor between six and eight weeks (Table 2).
A similar result was also found in concentrations of 0.8 (K2 group) and 0.3 mg/L (K3 group), for which there was a decrease in SOD levels as the duration of exposure increased (Table 2). The SOD levels were significantly higher for two weeks of exposure compared to the longer exposure time: after the second week, the SOD levels did not change significantly. At the lowest arsenic concentration in accordance with the permissible quality standard concentration (0.01 mg/L), SOD levels in the first two weeks were found at the highest level (2.50 ± 0.46 U/mL) then decreased afterward.
H2O2 is one of the oxidative compounds observed in this study, and the levels of uterus H2O2 after exposure to arsenic at various concentrations and durations are presented in Table 3. Our data suggested an increase in H2O2 levels at each additional exposure time in every arsenic concentration group. The mean H2O2 level in the control group after eight weeks was 2.33 ± 0.07 μM. This figure was significantly different from the other treatment groups (p < 0.0001). The multivariate test also showed a significant difference in the mean H2O2 levels between each treatment group (Table 3).
Table 4 presents the TNFα levels after exposure to arsenic in all treatment groups. Drastic spikes in all concentration groups were observed in the six- and eight-week exposure groups. These data suggest that the uterus inflammatory response to arsenic exposure through the vulva increased rapidly when arsenic exposure lasted more than 4 weeks. Overall, there were significant differences between the treatment and control groups (p < 0.0001). Post-hoc tests in all concentration groups also showed significant difference between the two- and four-week groups and the six- and eight-week groups (Table 4).
The uterus tissues from all animals were stained with HE and observed with 400×magnification. The total number of inflammatory cells in each group and statistical analysis test results are presented in Table 5. There was a significant difference of the number of inflammatory cells in arsenic exposure between length of exposures in all concentration groups (p < 0.0001). A further test using Duncan’s multiple range test found that there was no difference in inflammation at 5 mg/L arsenic exposure between two, four, and six weeks. The difference was only seen after eight weeks exposure. The arsenic concentration of 0.8 mg/L exposed through the vulva did not significantly differ between two, four, six, and eight weeks of treatment duration. At a concentration of 0.3 mg/L, there was a difference between two and four weeks of exposure and six and eight weeks of exposure. The 0.01 mg/L concentration group did not show any significant differences in any exposure time.
H&E staining on the uterus tissue suggested that inflammation occurred in the lining of the endometrium, myometrium, and perimetrium (Figure 1). Inflammatory cells were also found in the uterus fatty tissue. The representation of the histopathology of inflammatory cells in the uterus lining from all groups are presented in Figure 1.
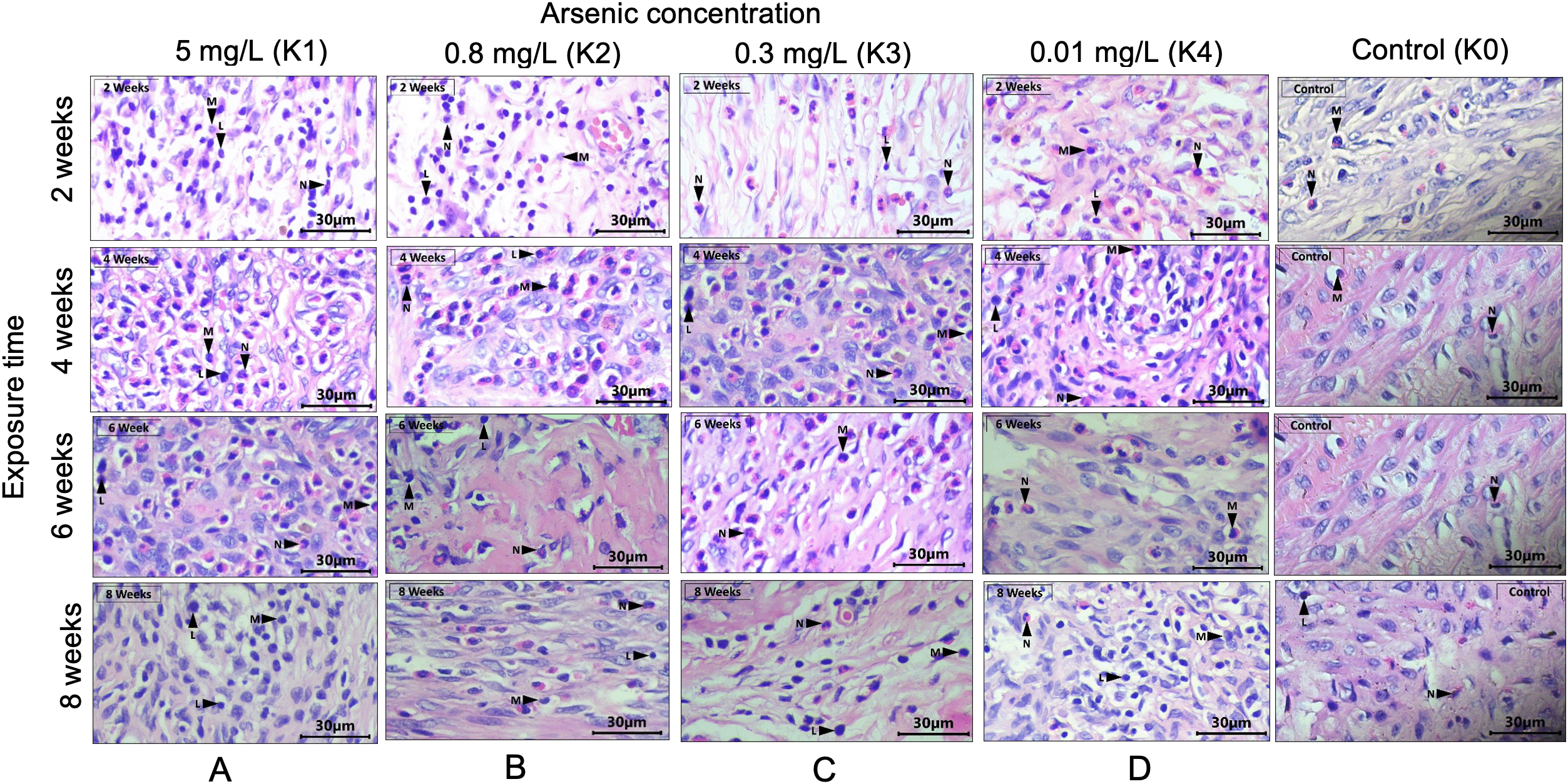
(A) infiltration of inflammatory cells occurred in all treatment groups. Inflammatory cells were found in the endometrium, myometrium, and perimetrium and reached the fat tissue. (B) Infiltration of N, M, and L occurred in all treatment groups (2, 4, 6, and 8 weeks). The inflammatory cells reached the myometrium layer, perimetrium layer, and fat tissue. (C) Infiltration of inflammatory cells in the control group was dominated by N. In the treatment group, the inflammatory cell infiltration contained N and M and also L. Inflammatory cells occurred in the lining of the endometrium, myometrium, and perimetrium. (D) Infiltration of inflammatory cells was found in all treatment and control groups. Most M and L were found in the treatment at 4 and 8 weeks. The inflammatory cells also reached the perimetrial lining. N: neutrophils, M: macrophages, L: lymphocytes.
Once absorbed by the vulva and vagina, arsenic seemed to increase its oxidative effect in the uterus. This is supported by increasing MDA and H2O2 levels and decreasing SOD levels in the uterus of experimental animals. Arsenic exposure rapidly stimulates the formation of intracellular reactive oxygen species (ROS). The ROS formed causes changes in how cells work. The contaminated cells might carry epigenetic modifications and changes in signaling pathways. ROS are even capable of causing direct oxidative damage to molecules. The induction of ROS production by arsenic in cells occurs via the mitochondrial electron transport chain and exerts a toxic effect on the mitochondria by inhibiting the activity of succinate dehydrogenase and releasing the oxidative phosphorylation pair through the production of superoxide anions to form other ROS.38,39 Another mechanism occurs through the deployment of nicotinamide adenine dinucleotide phosphate, which is oxidized (Nox). Nox is a membrane enzyme that produces ROS as a result of arsenic exposure. Nox accelerates the formation of superoxide anions. Another factor is that the metabolism of arsenic itself triggers the formation of ROS in cells, such as singlet oxygen, H2O2, superoxide anions, and SOD.38,39
Our data also suggested that the arsenic exposure at all concentrations induced the uterus MDA levels from a two-week exposure except for the 0.01 mg/L concentration. At this standard concentration, when exposed for two weeks, it did not cause oxidative effects. However, when the exposure lasted more than two weeks, the oxidative effect could still be observed. Arsenic exposure caused oxidative stress to the uterus in experimental animals after two weeks, indicated by the oxidative biomarker of MDA. MDA is a marker of oxidative stress derived from the end product of lipid peroxidation produced by free radicals.40 Our data suggests that the longer the exposure time and the higher arsenic concentration, the higher the ROS levels.
Slightly different from MDA, arsenic exposure induced H2O2 production even for the lowest concentration (0.01 mg/L). The permissible quality standard concentration of arsenic could increase the H2O2 level after two weeks exposure. The results also showed a decrease in SOD levels. SOD is an antioxidant defense enzyme, being the first line of defense against the occurrence of OS. SOD also acts as an enzyme detoxifying ROS and eliminating free radicals.41,42 Our data suggested arsenic exposure increased ROS and decrease the level of SOD. This condition could increase lipid peroxidation, endometrial cell growth and adhesion, and increase the formation of macrophages leading to an imbalance between antioxidants, especially SOD and ROS.43 This imbalance disrupts angiogenesis and blood supply, and causes persistent inflammation.43
Our present study also found that arsenic at the quality standard limit, when exposed continuously for more than two weeks, could cause oxidative effects. This caused the emergence of more ROS in the uterus, leading to infiltration of more macrophages. The higher the arsenic level and the longer the exposure, the higher the number of macrophages in the uterus tissues. Macrophages are part of the innate immune system, coordinate the process of the adaptive immune responses, restoring tissue homeostasis, inflammatory processes, and repair activities, and are also considered to be pro-inflammatory immune cells.44 The presence of macrophages could activate TNFα, a pro-inflammatory agent capable of regulating many aspects of macrophage function. TNFα can be released rapidly following toxicity, infection, or exposure to lipopolysaccharides and is considered to be a major regulator of pro-inflammatory cytokine production. Apart from pro-inflammatory cytokines, TNFα also enhances lipid signal transduction mediators such as prostaglandins and platelet-activating factors.45 Therefore, TNFα is considered to be the main cause of the activation and recruitment of inflammatory cells and plays an important role in chronic inflammation.45 Arsenic induces ROS production by changing antioxidant enzymes such as catalase and SOD to trigger the appearance of lymphocytes in inflamed tissues.46
Inflammation is a protective response that aims to protect tissues from becoming necrotic, eliminate the causes of infection, and stimulate tissue healing and repair processes. When inflammation occurs, neutrophils will appear as the body’s first defense cells against injury or infection. Macrophages will help the process of eliminating infection and damaged tissue through the process of phagocytosis. Lymphocytes play a role in chronic inflammation.47,48 Chronic inflammation occurred in the arsenic exposure groups with 0.5, 0.8, and 0.3 mg/L concentrations. This chronic inflammation was characterized by the appearance of abundant lymphocytes. The appearance of this accumulation of leukocytes is due to tissue injury resulting from a long-term inflammatory response.49 Although macrophages and lymphocytes were found in animal exposed to 0.01 mg/L arsenic, the number was significancy smaller. However, exposure for more than two weeks also could present inflammation responses. Our data also found that neutrophils were present in the uterus in all treatment groups. Inflammatory cells were also present in the control group in very small numbers and only neutrophils and macrophages were present. Neutrophils are the first cells present when there is tissue injury and infection and are associated with the body’s defense against infection and other inflammatory processes and it is also known as the first responder and constant defender.47
Our data suggest that continuous and long-term immersion of animal vulva with water containing arsenic even at the permissible concentration (0.01 mg/L) could cause inflammation of the uterus tissues. Arsenic at 0.3, 0.8, and 5 mg/L could cause chronic inflammation of the uterus after a two-week exposure. Arsenic exposure was able to increase the uterus MDA levels even at 0.01 mg/L when exposed for more than two weeks. Arsenic increased the levels of H2O2 at all concentrations after a two-week exposure. In contrast, arsenic also induced the ROS by reducing the levels of SOD in uterus. Arsenic could increase the level of TNFα at any concentration after six and eight weeks of exposure.
Figshare: ‘Damage to the uterus due to arsenic exposure to the vulva via oxidative stress (MDA, SOD and H2O2) and inflammatory (TNF-α) pathways of female Sprague-Dawley rats’. DOI: https://doi.org/10.6084/m9.figshare.21774989. 50
This project contains the following underlying data:
Master Table.xlsx [Table containing the raw data of the study].
Figshare: ARRIVE checklist for ‘Damage to the uterus due to vulva exposure to arsenic via oxidative stress (MDA, SOD and H2O2) and inflammatory (TNF-α) pathways of female Sprague-Dawley rats’. https://doi.org/10.6084/m9.figshare.21774989. 50
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Authors would like to thank the Indonesian Ministry of Health for providing the fund for this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Life sciences
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environment pollution; heavy metal ions; Biomonitoring; Multielemental analysis of biological samples
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Jan 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)