Keywords
Prevalence, Healthcare Associated infection, Antibiotic
Prevalence, Healthcare Associated infection, Antibiotic
Healthcare-associated infections (HAIs) constitute a major global public health concern worldwide with a high attributable morbidity and mortality.1
They lead to a heavy economic burden on health systems by extending the length of patient’s hospital stay in healthcare settings, increasing medical care costs and prescribing supplementary antibiotics, thus increasing the risk of antimicrobial resistance problem.2
This concern represents a real health threat, especially in the developing countries with a higher morbidity and mortality compared to developed countries. The risk of contracting a HAI during hospital stay is multiplied by 2 to 20 times in developing countries with a prevalence that can exceed 25% in some countries.3
This problem is still largely underestimated in these countries because of the lack of surveillance data and epidemiological studies.4
There are limited data on HAIs available in Tunisia. According to the latest published results of the national nosocomial infection prevalence survey conducted in 2005 “NosoTun-2005”, the prevalence of HAIs was of 13.2%.5
Conducting regular cross-sectional point prevalence surveys to have punctual and rapid baseline information about HAIs is recommended and constitutes a good alternative to overcome the problem of unavailable continuous prospective surveillance system in hospital settings.6
Before instauration of a prevention strategy program at a healthcare institution we need to assess the magnitude of the HAIs problem and to identify the high-risk population to establish priorities of infection prevention and control.
This study aimed to estimate the prevalence of HAI among adult patients at the Charles Nicolle Hospital (CNH) of Tunis and to identify the main associated factors as well as to estimate the frequency of antibiotic use.
We conducted an observational cross-sectional (point prevalence) survey in all the adult wards (medical and surgical specialities) in the tertiary university CNH of Tunis from October to December 2018. Data collection was finalized within the same day for each ward, with a single passage per ward.
All patients hospitalized for 48 hours or more and having an age >18 years were included in the survey. All patients which were effectively present (including any patient absent from bed for further examinations, surgery, or specialized consultation) at the day of the survey were included. Patients consulting at outpatient departments, outpatient dialysis, and emergency rooms were not included.
The Centers for Disease Control and Prevention (CDC) Point Prevalence Survey (PPS) protocol was used as a methodological guideline. HAI was retained if the patient had a clinically active (having symptoms of infection present on the survey date, or if signs and symptoms were present previously and the patient was receiving treatment on the survey date) and/or microbiologically confirmed infection at the survey day (based on results of microbiological exams requested by the medical staff from the patient's file). We adopted the definition of different anatomic HAI sites proposed by the CDC.7
Data collection was carried out using the medical records and by interviewing the nurse and the medical staff if necessary. The identification and the affirmation of the nosocomial source of any type of infection was confirmed by the medical staff with reference to the CDC definitions criteria.
The information was collected using a standard data collection form per patient. It consisted of two main parts:
• A part for the identification characteristics of the patient; the reason for hospital admission, medical and surgical risk factors such as tobacco and alcohol consumption, immunosuppression, neutropenia, diagnostic category (medical or surgical), infection on admission, diabetes, hypertension, history of a surgery act during the 30 days before the survey, inserted invasive devices and ATB use. Factors related to the surgical interventions such as the context of the surgery (in emergency or programmed), and the American Society of Anesthesiologists (ASA) physical status score1 (The ASA classification and peri-operative risk8) and the surgical wound classification (class I: Clean; class II: Clean-Contaminated; class III: Contaminated; class IV: Dirty-Infected) were also collected. This first part was filled for all included patients.
• A second part that has been filled only for patients with one (or more) HAI(s) with information regarding the site of infection and microorganism(s) isolated.
Training sessions for all investigators were organized before the start of data collection. The training was done to explain the aim of the study, the practical modalities of study conduction and to represent the data collection form.
Heads of the different adult wards have been officially contacted by the hospital management to inform them about the study. A referent staff (medical or paramedical) has been appointed in each ward to ensure the smooth running of the study and to facilitate data collection and access to information for investigators.
A list of all eligible patients aged > 18 years and who are hospitalized for at least 48 hours in each ward was prepared in advance (at the day of the survey) by the referent staff before starting data collection by the investigators team.
Our study follows the SAGER guidelines for reporting sex and gender information. Sex and gender differences were not taken into consideration for the design of the study. Adequate representation of males and females was not possible because it was a cross sectional study without sampling and all eligible patients were included in the study regardless of sex. The information regarding the sex of participants was collected from records. Sex-based analyses was reported.
All statistical analysis were performed using SPSS Statistics software version 23 (IBM Corporation, Armonk, NY).
The statistical unit of analysis was the infected patient with one or more site of HAI.
Qualitative variables were presented as simple frequencies and relative frequencies (expressed into percentages). The mean (± the standard deviation) or the median with the interquartile range, as well as the extreme values were estimated to summarize quantitative variables.
The prevalence of HAIs was estimated with presentation of the 95% CI.
Proportions and means were compared using Chi square test (Fisher's exact test if not applicable) and Student’s t-test for independent groups, respectively.
Independent risk factors of HAIs were identified by multivariate analysis using binary logistic backward stepwise regression (adjusted OR [AOR]; 95% CI; p).
In the univariate logistic regression, measures of association were expressed as brut odds ratios (ORb) with 95% confidence intervals (CIs).
The selection of the explanatory variables to be introduced into the initial model of the multivariate analysis was made by taking into consideration the significance level p ≤ 0.20 at the stage of the univariate analysis and by introducing variables having a clinical relevance (not significant in the univariate analysis) in the initial model and which are known as confounding factors. The significance level was fixed to p ≤ 0.05.
All included participants were informed about the purpose of the study and have given their verbal consent to be included in the study. All medical and paramedical staff who were interviewed to have access to patients’ information were informed about the aim of the study and have given their verbal consent to collaborate with the investigators team. They were also informed about their right to refuse participation or drop out at any moment of the study collection. All collected information and data analysis was confidential and anonymous during and after data collection.
No blood samples were taken or procedures performed on the participants for research purposes. This survey was carried out as part of activities to limit HAI in the CNH. The study protocol was presented to the medical committee of the hospital and was approved in September 2018 before the start of the investigation. Considering that the data collection had to be completed in a single day during a single visit to each ward, since it was a point prevalence survey, and given the small number of investigators and most of the hospital departments are very large with several units, we preferred to obtain verbal consent to facilitate data collection in the field. The verbal consent was discussed and approved by the committee.
Table 1 summarizes patients’ baseline characteristics. Overall, 261 patients were included, with 145 women (55.6%) and a sex ratio (F/M) = 1.25. The median age was 53 years (IQR = [39-65 years]), with extremes varying between 19 and 90 years old. In total, 69 patients were aged above (≥) 65 years (26.4%). Antibiotic treatment was used in 84 (32.2%) cases. 13 (5.0%) patients resided in an intensive care unit (ICU) unit on the day of the survey. 52 (19.9%) underwent a surgery during the last 30 days before the survey date. 76 (29.1%) patients had at least one invasive device (ID) inserted during the last 7 days prior the investigation date.
A total of 34 patients having at least one active HAI were identified among eligible patients, which represents a rate of infected patients of 13% (95% CI [9.2%–17.0%]). The frequency of HAI was not significantly associated with sex (16.4% (n = 19) among males vs. 10.3% (n = 15) among females; p = 0.15). The characteristics of these patients are summarized in Table 2.
A total of 36 sites of HAIs was identified which represents a rate of HAI site prevalence of 13.8% (95% CI [10.13%–18.5%]). 2 patients were identified with 2 active HAIs.
The most frequent types of reported HAI were urinary tract infections (n = 12, 33.3%) followed by surgical site infections (n = 7, 19.4%) and pneumonia (n = 7, 19.4%).
Of all HAIs, 15 infections were microbiologically documented (15/36 = 41.6%). Pseudomonas aeruginosa and Escherichia coli were the most frequently documented microorganisms with equivalent frequency of 26.6% for each one.
Univariate analysis revealed that alcohol use (ORbrut = 3.8; p = 0.03), having community infection at admission (ORbrut = 3.3; p = 0.001), having at least one invasive device during last 7 days prior to the survey date (ORbrut = 5.9; p < 0.001), surgery during last 30 days prior to the study date (ORbrut = 2.5; p = 0.01) were found to be significantly associated with HAIs.
Risk factors found to be independently associated with HAIs in multivariate analysis, were: having hypertension (ORadjusted = 3.3; p = 0.008), alcohol use (ORadjusted = 5.2; p = 0.01), being infected at admission (ORadjusted = 2.8; p = 0.01), having at least one invasive device during last 7 days prior to the survey date (ORadjusted = 3.5; p = 0.004) and undergoing surgery 30 days prior to the study date (ORadjusted = 2.6; p = 0.03) (Table 3).
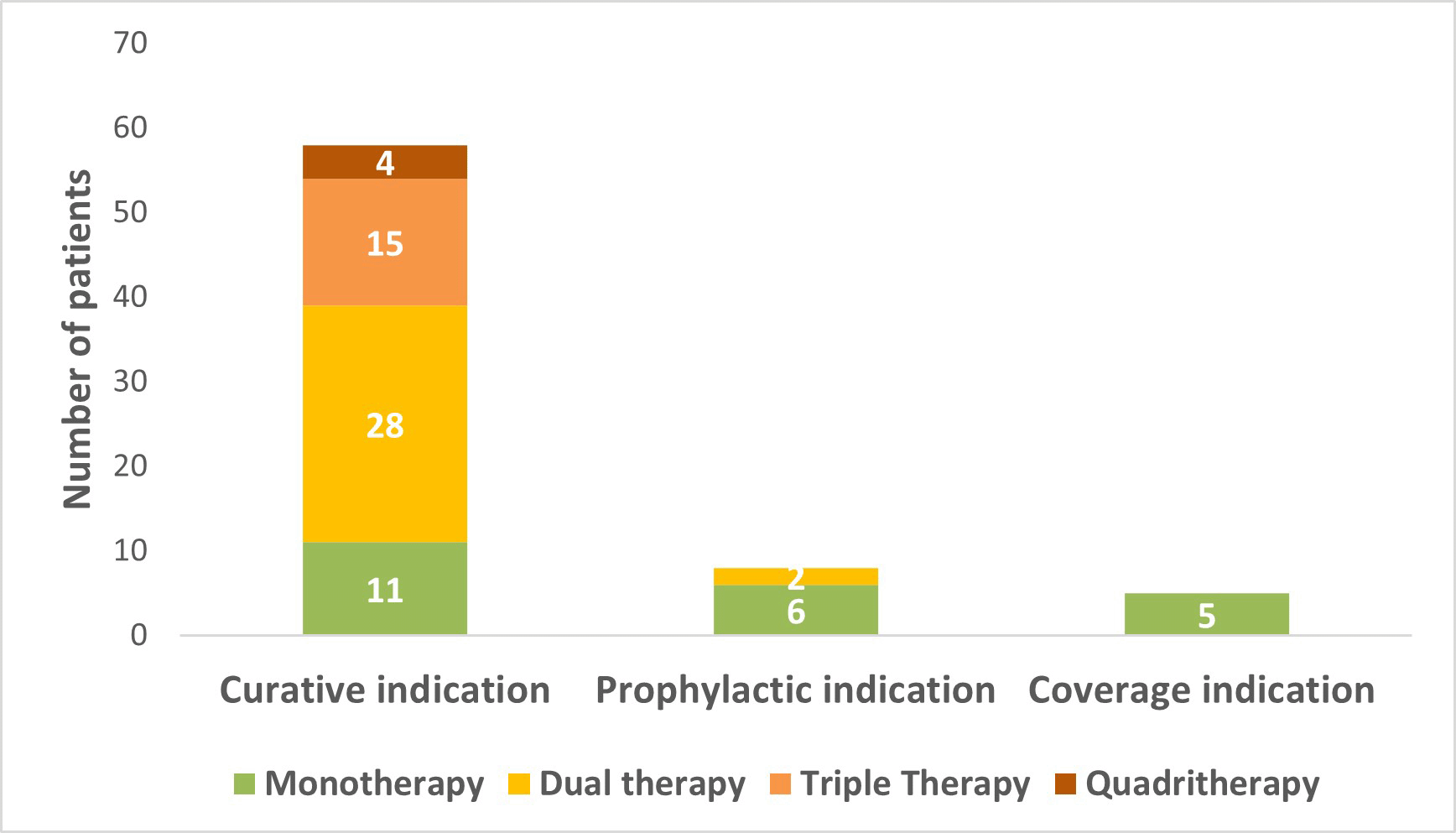
The prevalence of antibiotic use was 32.2% (IC 95% [26.3, 37.9], n = 84/261) cases at the survey date. There was a significant difference of antibiotic use by sex (38.8% (n = 45) among males vs. 26.9% (n = 39) among females; p = 0.046). There was no significant association between antibiotic use and age categories (30.7% (n = 59) among]18-65[years vs. 36.2% (n = 25) among patients ≥65 years); and diagnostic category at admission (33.9% (n = 64) for medical patients vs. 28.6% (n = 20) for surgical patients). 35 patients (41.7%) were under one agent, 30 patients (35.7%) were under two agents and 19 patients (22.6%) were under 3 or more agents. In most cases (81.7%, n = 58/71), antibiotic agents were prescribed for a curative indication (see Figure 1).
Cefotaxime (Claforan®) (17.6%, 27/153), Amoxicillin/clavulanate (Augmentin®) (9.1%, 14/153) and Carbapenem (Tienam®) (8.5%, 13/153) were the most frequently prescribed antibiotic agents.
A total of 52 patients (19.9%) had been operated on during the last 30 days before the survey with a mean age of 54.1 (±17.8) years, of which 26 were female (50.0%). ASA score was specified for only 36 patients, of which 55.6% were classified ASA 1, 33.3% ASA 2 and 11.1 % ASA 3. According to the surgical wound classification, 67.3% (n = 33) were class 1 (clean) and 12.2 % (n = 6) were class 4 (dirty-infected).
Among all the operated patients, the use of antibiotic prophylaxis was specified for only 45 patients. Among these patients, 20 (44.4%) have received antibiotic prophylaxis before ongoing the surgery intervention. Half of them (n = 10) have received antibiotic prophylaxis for a duration ≥ 24 H. Monotherapy was prescribed for most of these patients (n = 16/18, 88.9%) and a bitherapy (2 antibiotics) was prescribed for 2 patients (n = 2/18, 11.1%). Results disaggregated by sex are represented in Table 4.
The prevalence of HAI among the operated patients was 23.1% (n = 12).
The prevalence of infected operated patients did not differ significantly according to the sex distribution (p = 0.32), to the operating context (urgent or programmed) (20.0% versus 24.3%; p = 0.73), to the surgical approach (p = 0.6, to the ASA score (p = 0.5 and to the use of antibiotic prophylaxis (p = 0.5). Only the surgical wound classification was significantly associated with HAI (p = 0.03).
A total of 13 infection sites were documented. Superficial surgical site infections were the most common (n = 6, 46.1%) (Figure 2).
A total of 7 microorganisms were isolated among 7 bacteriological samples done (see Table 5).
The control of the infectious risk is considered as an indicator of quality of care. HAIs represent one of the serious healthcare-associated adverse events at health care structures that threaten patient safety.9
The principal findings of our point prevalence survey were that approximatively one patient out of eight had at least one active HAI, and one among three patients was under at least one antibiotic agent on the survey day. The most frequently reported site infections were urinary tract infections followed by surgical site infections and pneumonia.
Comparing to other studies conducted in Tunisian hospitals, a similar prevalence was found at Farhat Hachad Hospital in 2012 with a prevalence of HAIs of 12.5%.10 Lower prevalences of 10.9% and 9.1% was reported in two university hospitals in Sfax in 2017 and 2019, respectively.11,12
Another study carried out in Sahloul Hospital based on data collected from prevalence surveys conducted between 2012 and 2020 revealed a total prevalence of 10.8%.13
The higher prevalence found in our study can be explained by the relatively higher number of wards specialized in interventional procedures (2 large general surgery departments, 2 adult intensive care units, a gastroenterology department), which may increase the infectious risk.
Our results were close to those estimated in other studies carried out in low- and intermediate-income countries with an estimated overall HAI prevalence of 15.5%, according to a meta-analysis conducted in 2011.1 It reached 28% in some Saharan African countries.14,15 Our prevalence was considerably higher compared to developed countries, in particular European countries with a global prevalence of 7.5%.16
Theses discrepancies may be due to the inexistence of a monitoring system and control of these infections in health structures, especially in developing countries. Such systems ensure the application and the respect of hygiene rules by all health care staff, and the availability of all the necessary materials for hand hygiene. All these conditions are not always available in hospitals in developing countries, which may explain the higher prevalence of HAI in these countries compared to other developed countries.
In our study, independent risk factors identified as associated with increased HAI risk were having hypertension, consuming alcohol, being infected at admission, having at least one invasive device during last 7 days prior to the survey date and undergoing surgery 30 days prior to the study date.
Indeed, hypertension is a predisposing factor to atherosclerosis, which in turn leads to a stimulation of the immune system with its two cellular and humoral components.17
On the other hand, alcohol causes a secondary immune deficiency by a molecular mechanism, which increases the risk of infections in general and HAIs in particular.18,19
Like our fundings, several studies have shown that a history of surgery during the 30 days prior to admission and recent exposure to invasive medical devices are risk factors for HAIs.12,13,20,21
In general, a special attention should be given to immunocompromised and infected patients who constitute a population with a very high risk of contracting this type of infections.
The most important avoidable risk factor identified in our study was the exposure to invasive devices.
The risk of contracting device-associated HAIs is known as preventable.22 It depends essentially on the degree of respect of hygiene rules, the quality of their daily care and their insertion time before remove. Thus, their indication, their number and their insertion time must be limited as possible.
Implementing evidence-based practices using bundles in infection prevention can improve patient safety and reduce HAIs related to invasive devices.22,23
The most frequently identified sites of infection in our study, were urinary tract infections, pneumonia and superficial surgical site infections. This result joins that found in several other studies in Europe,24–26 Asia and Africa.4 Similarly, a study conducted in Sfax in the two university hospitals, Hedi Chaker and Habib Bourguiba, Tunisia, in 201711 found similar results.
Escherchia coli and Pseudomonas aeroginosa were the most frequently isolated germs with a frequency of 27.8% respectively for each germ, and in third place Acinetobacter baumannii. These Gram-negative bacteria are the most frequently isolated type according to the literature. In fact, this result is in line with a systematic review study conducted in 2019 that included European, Asian and African studies, which found the same results.4 These two types of germs have also been identified in other Tunisian surveys.10,11,27
Gram-negative bacteria have a great capacity of acquisition of genes coding for antibiotic resistance, which may explain their frequent incrimination in HAIs.28
Otherwise, health care workers represent a main component in the transmission process of these infections. The main axis of prevention that can be targeted is improving hand hygiene compliance among health care workers which have been demonstrated being significantly effective in reducing the nosocomial infection risk.29
Providing regular training and reminders using posters at the workplace settings can be very useful. Raising the degree of awareness about the importance of the problem and the necessity of their implication as a principal actor on prevention strategy, is needed to reduce the occurrence of these infections. Continuous availability of alcohol-based hand rubs at point of care is also a very important component to motivate hand hygiene practice.30
The prevalence of antibiotic use, in our study, was alarming. Almost one patient out of three was under antibiotic therapy on the day of the survey and third generation Cephalospoins (Claforan) were the most frequently prescribed antibiotics. This prevalence was comparable to that estimated in some developed countries (overall prevalence of 35%, ranging from 21% in some countries to 55%).24,31 More than half of patients receiving antibiotics were under two or more agents. Rational use of antibiotic combinations and reduction of unnecessary antibiotic prescribing is needed. Creating an antibiotic stewardship program is very important to limit the development of antibiotic resistance in health care facilities.
In the other hand, our survey enabled us to estimate in a simple and inexpensive way the frequency of HAIs and antibiotic use among adult wards at the Chrales Nicolle Hospital.
Point prevalence survey is considered as a useful, rapid, and cost-effective method to quantify the magnitude of HAI problem and target interventions priorities, particularly in hospitals with limited resources.32,33
It was an opportunity to raise awareness and remind health care workers about the importance of the nosocomial infectious risk and to increase their motivation and their adherence to the prevention programs.
Incidence surveys are the gold standard tool used in HAIs surveillance, but they require much more time, resources, and costs. Repeated point prevalence surveys are a good alternative method for measuring HAIs trends over time and to assess the impact of interventions and control programs and actions.34
Controlling HAIs is a long-term process that requires the implication of multidisciplinary staff (clinicians, microbiologists, epidemiologists, hygienists, pharmacists, and administrative agents), and needs a good coordination of their interventions with a clear definition of each one tasks and responsibilities. But it supposes a minimum of local possibilities, in relation to the premises, materials, organization of care and a certain degree of agreement about behaviors and practices.
The prevalence rates of HAIs and antimicrobial use in adult wards at CNH were relatively high. Hospital infection prevention and control measures need to be strengthened to reduce the burden of HAI at the institutional level. Infection control priorities must essentially target urinary tract infections, surgical site infections and pneumonia.
An infection prevention and control committee, as well as the development of an antibiotic stewardship program with continuous monitoring using repeated prevalence surveys, must be implemented to limit the frequency of these infections effectively.
While further research is necessary by estimating the incidence of these infections prospectively over time to understand the principal risk factors and evaluating their real economic impact by estimating the direct cost of HAIs.
Harvard Dataverse: Healthcare associated infection-Tunisia, https://doi.org/10.7910/DVN/00BSW9. 35
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)