Keywords
study within a trial, pen, participant information, brief information leaflet, recruitment, factorial, randomised controlled trial, persistent physical symptoms
This article is included in the Studies Within A Trial (SWAT) collection.
study within a trial, pen, participant information, brief information leaflet, recruitment, factorial, randomised controlled trial, persistent physical symptoms
Despite substantial funds being invested in the UK and internationally, many randomised controlled trials (RCT) in healthcare fail to recruit on time and to budget.1 Many strategies are used by trialists to improve recruitment; however few such interventions have been rigorously evaluated.2,3 Trials embedded in real-life ‘host’ trials (also known as ‘Studies within A Trial’ [SWATs]) are the most robust way of evaluating such interventions.
There is some evidence that using a pen as a nonmonetary incentive increases response rates and time to response for trial follow-up questionnaires.3,4 The primary theory underlying the use of pen incentives is that of reciprocation.5–8 In the context of trial recruitment, offering a potential participant a gift such as a pen may make the person more likely to take up the trial invitation to enrol. It is also possible that the convenience of having a pen to hand upon receipt of the invitation may increase the likelihood of the forms being completed, or the timeliness of return. One U.S. trial embedded in an observational study showed that including a pen with the study logo to a questionnaire mailed to women who had previously not responded, significantly improved recruitment rates.9 However, a SWAT embedded into a RCT testing yoga for older adults with multimorbidity found no improvement in randomisation or response rates with the inclusion of a pen incentive within postal recruitment packs.10
A common method of recruiting participants from general practices and other registries into trials is to send letters to potentially eligible patients inviting them to participate, along with the trial Participant Information Sheet (PIS). PISs are lengthy and increasingly complex11 and being asked to read such a large document may act as a deterrent to potential participants. A shorter PIS (or participant information leaflet (PIL)) may be more appealing initially, as it is likely to provide a more manageable volume of information.12 A Cochrane review of recruitment interventions identified two trials that have evaluated a brief PIL compared with a full PIS,2,12,13 and found the brief PIL makes little or no difference to recruitment compared with a full PIS. RD = 0% (95% CI = -2% to 2%); GRADE: moderate. However, this needs replicating in different populations.
In this study we aimed to evaluate the effectiveness and cost effectiveness of a brief PIL (provided in addition to a standard length PIS) and a trial logo branded pen on recruitment and response rates in the Multiple Symptoms Study 3 (MSS3) host trial. The SWAT was part of the PROMETHEUS programme (MRC MR/R013748/1) designed to identify effective and cost-effective methods to improve recruitment to and retention in trials, and to identify if it is possible to routinely embed SWATs within trials.
The MSS3 SWAT was approved alongside the host MSS3 trial by the North West – Greater Manchester Central Research Ethics Committee (18/NW/0422). In line with that approval, patients were not informed about the SWAT and therefore, informed consent was not obtained. The MSS3 protocol was registered on ISRCTN (57050216) and has been published.14 The SWAT was registered with the Northern Ireland Network for Trials Methodology Research SWAT repository (SWAT137). The paper is reported in line with the Trial Forge Guidance: template for reporting the results of randomised Studies Within A Trial.15
We undertook a 2×2 factorial randomised SWAT. Potential MSS3 participants were identified by GP practices through computer searches and record screening. Practices were located in Yorkshire and the Humber, Greater Manchester, Newcastle and Gateshead, and Northwest London. All identified individuals that were to be invited to participate in MSS3 were randomised to one of four arms, determining how they would be invited to participate:
The pen was branded with the MSS3 logo and colours (Figure 1). The brief PIL consisted of an A4 sized sheet printed on high quality paper in colour and folded into three, in a leaflet style (Figure 2). It was designed to provide a more succinct and easy to read summary of the MSS3 trial than the standard PIL. The information and format were reviewed by the patient representative on the MSS3 Trial Management Group. It was provided alongside the standard PIL in the recruitment pack. The invitation letter explained that the brief PIL provides a summary of the research in order for the potential participant to decide if they might be interested in the study and that the standard PIL provides more details should they wish to read this before returning the form, but that they will have the opportunity to discuss the study with a member of the research team and ask questions later.
The primary outcome was the recruitment rate, being the proportions of participants in each SWAT intervention group who were randomised into the host trial. Secondary outcomes were the proportion of patients who returned an expression of interest form, the proportion of patients who returned an expression of interest form but were not randomised due to a) ineligibility or b) non-consent, the time taken to respond to an invitation, and the cost-effectiveness of the interventions. The characteristics of participants returning an expression of interest and of those randomised into the host study were also collected. Data on sex were self-reported.
We did not undertake a formal power calculation to determine the sample size, since this was constrained by the number of patients being approached in the MSS3 host trial. However, based on response rates achieved in two preliminary studies we estimated we would need to invite 4888 patients in order to recruit 376 to the trial, representing a recruitment rate of about 8%. This would provide 80% power to identify a 3% absolute difference between the groups in recruitment rate if one existed. A simple multiple comparison adjustment was applied, using a significance level of 2.5%, which would allow us to test both interventions.
Individual-level randomisation using randomly-permuted blocks of length four or eight, equal allocation ratio and stratification by GP centre was used to allocate participants to receive one of the four packs. The allocation lists were generated by a CTRU statistician and shared only with the CTRU staff preparing the invitation packs, who were independent of the CTRU staff who processed the invitation responses.
All recruitment materials were placed in pre-stamped envelopes. The packs were placed in order of the random allocation list and then numbered sequentially before being sent to the practice. Practice staff were informed to label the recruitment packs with patient addresses in the sequential order that the researcher had prepared them. Patients did not know that they were part of a trial testing recruitment interventions so were blind to the SWAT hypothesis. CTRU staff undertaking MSS3 trial recruitment were blind to the SWAT group to which patients were allocated. It was not possible to entirely blind practice staff to the interventions as it will have been clear that some packs contained pens.
Analyses were conducted on an intent-to-treat basis, including all participants in the intervention group to which they were randomised. Participants entering the recruitment process following a reminder invitation were not included in the SWAT analysis.
Factorial analysis ‘in the margins’ was used to investigate the main effects of the brief PIL intervention and the pen intervention. The assumption of no interaction between factors was tested by fitting multivariable regression models including interaction terms. There were no other covariates included in the models.
The primary outcome of randomisation into the host trial was assessed using binary logistic regression. Results for each intervention are presented as odds ratios with 95% confidence intervals. The main model included fixed effects only; sensitivity analysis was conducted using random centre effects.
Cost-effectiveness was determined by calculating the number needed to treat (the inverse of the absolute difference in proportions with successful outcome between intervention and control) and multiplying it by the additional cost of the intervention compared with the standard invitation pack.
The time taken to respond to the initial invitation was determined by calculating the days between the date an invitation was posted and the date a response was received. Partial postage dates were imputed using the first day of the month. Negative or zero date differences were set to missing. Non-responders were censored at the date the study closed to recruitment. Median response times were calculated for participants returning an expression of interest. Data were assessed visually using Kaplan-Meier plots, and between-group comparisons made using Cox regression. Hazard ratios are reported for each intervention with corresponding 95% confidence intervals.
Summary statistics are reported for each of the four arms to which participants were randomised. Categorical variables are summarised by counts and percentages, and continuous variables using mean (SD) and median (range). Missing data are quantified. Inferential statistics are reported by way of main effects for each of the two interventions. Odds ratios and hazard ratios greater than one favour the intervention group; ratios of one are indicative of no between-group difference; ratios less than one favour the respective control group. For each outcome the brief PIL-pen interaction term is also reported. Analyses were conducted in R version 4.1.2.
Participant flow from the initial postal invitation to randomisation in the MSS3 trial is detailed in Figure 3. Recruitment took place between October 2018 to December 2021. A total of 108 GP practices participated in the recruitment process, with 6978 patients initially identified as potentially eligible for invitation to the MSS3 host trial. There were 32 patients whose eligibility status changed between initial GP search and the point at which invitations were posted, so a total of 6946 invitations were sent, of which 318 (4.6%) were randomised following an initial invitation. The SWAT analysis excludes participants who responded to a reminder invitation - there were 2530 reminders sent over the course of the recruitment period and a further 36 participants randomised. The total proportion recruited to the MSS3 host study following both initial and reminder invitations was 354/6946 (5.1%).
Participant numbers for factorial analysis ‘in the margins’ to determine average brief PIL and pen effects are presented in Table 1.
| Pen | No pen | Total | |
|---|---|---|---|
| Brief PIL | 1731 | 1736 | 3467 |
| No brief PIL | 1733 | 1746 | 3479 |
| Total | 3464 | 3482 | 6946 |
The proportion randomised per intervention group is shown in Table 2.
| N invited | N (%) randomised | Odds ratio (95% CI) | |
|---|---|---|---|
| Brief PIL | 3467 | 166 (4.8%) | 1.10 (0.88, 1.38), p = 0.403 |
| No brief PIL | 3479 | 152 (4.4%) | |
| Pen | 3464 | 145 (4.2%) | 0.84 (0.67, 1.05), p = 0.119 |
| No pen | 3482 | 173 (5.0%) |
Interval estimates for both interventions include the null value, consistent with the hypothesis of no effect on recruitment to the host trial. Sensitivity analysis was conducted using random GP practice effects. The intra-class correlation coefficient was 0.074 and the fixed-effect estimates were consistent with the primary model.
Of the 6946 invited participants, 1086 (15.6%) returned an expression of interest form. Proportions expressing interest in participating in the MSS3 host trial are shown by intervention group in Table 3.
| N invited | N (%) responded | Odds ratio (95% CI) | |
|---|---|---|---|
| Brief PIL | 3467 | 573 (16.5%) | 1.14 (1.01, 1.30), p = 0.041 |
| No brief PIL | 3479 | 513 (14.7%) | |
| Pen | 3464 | 563 (16.3%) | 1.10 (0.96, 1.25), p = 0.158 |
| No pen | 3482 | 523 (15.0%) |
There was evidence of a higher rate of return in the brief PIL group, with estimated odds of return 14% greater than participants not receiving a brief PIL.
The additional cost of including a brief PIL in the invitation pack was £0.50. Given the 0.4% increase in participants randomised (shown in Table 2), 239 brief PIL invitations would need to be sent at a cost of £119.50 to recruit one additional participant to MSS3. The additional cost of including a pen in the invitation pack was £0.41. Cost-effectiveness was not calculated for the pen intervention, given the lower proportion randomised compared to the group of participants not receiving a pen.
There were 768 SWAT participants (70.7%) who returned an expression of interest form but did not go on to be randomised to the main trial. Reasons for non-randomisation are provided in Table 4, by arm and overall.
| Reason | Control (n = 171) | Brief PIL only (n = 179) | PEN only (n = 190) | Brief PIL and pen (n = 228) | Total (n = 768) |
|---|---|---|---|---|---|
| Ineligible (screen 1)* | 87 (48.6%) | 82 (48%) | 103 (45.2%) | 88 (46.3%) | 360 (46.9%) |
| Could not be contacted** | 35 (19.6%) | 32 (18.7%) | 40 (17.5%) | 37 (19.5%) | 144 (18.8%) |
| Did not wish to proceed** | 21 (11.7%) | 15 (8.8%) | 17 (7.5%) | 13 (6.8%) | 66 (8.6%) |
| Ineligible (screen 2)** | 18 (10.1%) | 14 (8.2%) | 24 (10.5%) | 20 (10.5%) | 76 (9.9%) |
| Enrolment not scheduled** | 3 (1.7%) | 4 (2.3%) | 2 (0.9%) | 2 (1.1%) | 11 (1.4%) |
| Ineligible (screen 3)*** | 5 (2.8%) | 9 (5.3%) | 20 (8.8%) | 12 (6.3%) | 46 (6%) |
| Did not consent/did not attend*** | 10 (5.6%) | 15 (8.8%) | 22 (9.6%) | 18 (9.5%) | 65 (8.5%) |
More than half the participants expressing interest but not going on to be randomised to the host study were found to be ineligible at one of the screening assessments. Of the 65 participants who completed the full screening process but did not consent to be randomised, 16 (24.6%) stated they were no longer interested in participating. The remaining reasons were listed as “other” or missing; free text notes implied the majority occurred due to participant non-attendance.
For participants who returned an expression of interest, the median return time was 16 days. Eight GP centres had partial postage dates imputed with the first day of the month. Five observations were excluded due to missing time-to-event data. Kaplan-Meier plots are presented in Figure 4.
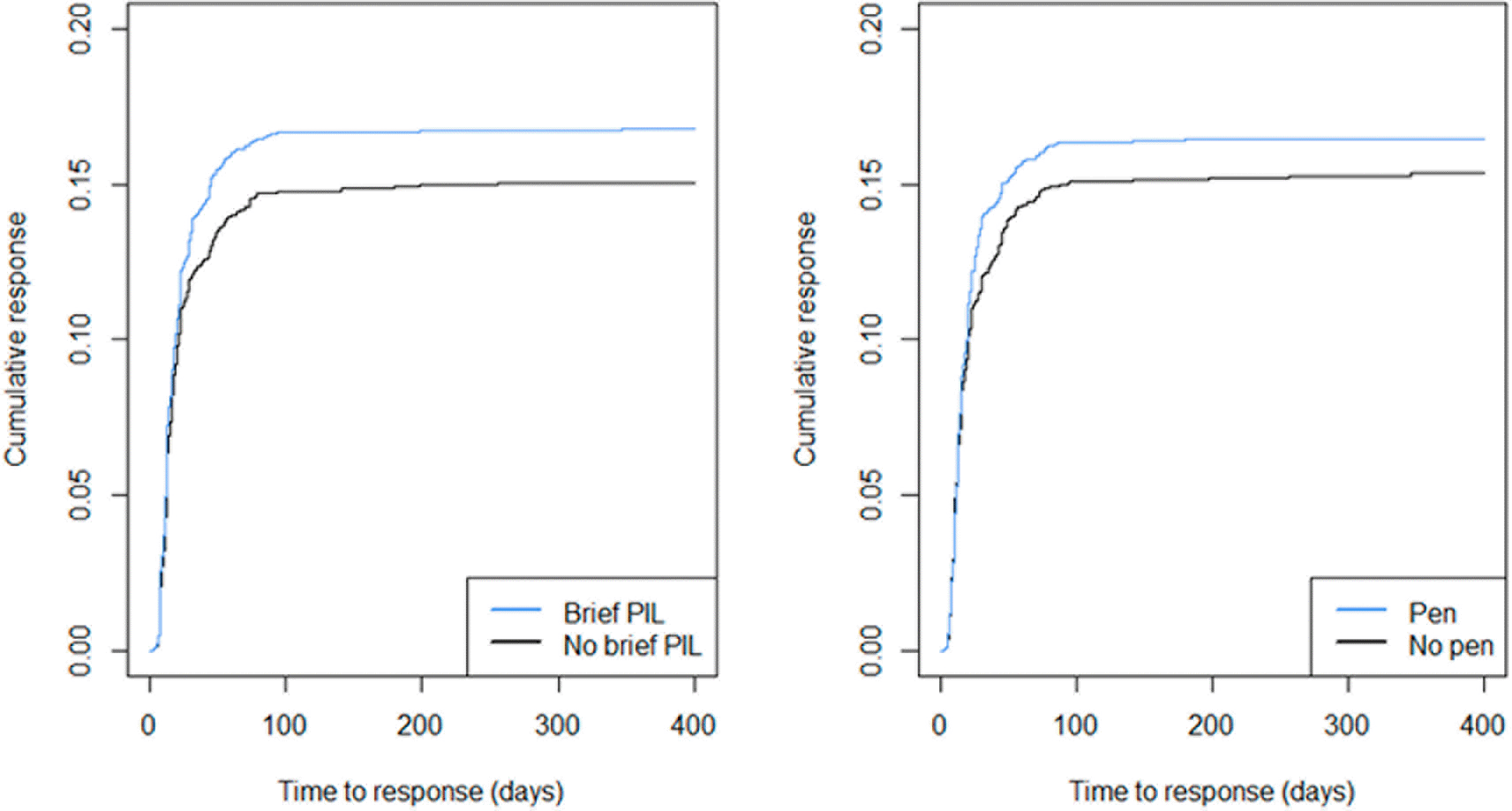
PIL = participant information leaflet.
For those who responded, the time to response was comparable between groups, but a higher proportion responded in the brief PIL and pen groups compared to their respective control groups. Hazard ratios are presented in Table 5. There was evidence of greater response rate (estimated 13% improvement) at any given time in the brief PIL group compared to no brief PIL.
Characteristics of participants returning an expression of interest form are provided in Table 6.
Demographic information for the group of participants successfully randomised is presented in Table 7. Age and sex distributions are comparable to the larger ‘expression of interest’ group.
We observed no significant evidence of a difference in the proportion of participants randomised to the MSS3 trial, in those receiving either a pen or brief PIL compared to control. We did observe a significantly higher response rate in those receiving the brief PIL compared to control, but this did not result in an increased randomisation rate in this group. Response rates for those receiving the pen were also higher than the control group, but this was not statistically significant. Response rates were overall lower than had been anticipated when designing the MSS3 trial. There was no difference in time to response between the groups. We did not observe any differences in the characteristics of those expressing an interest versus those randomised, although very little data were available for this comparison.
It is interesting that inclusion of the brief PIL resulted in better response rates but not randomisation rates. One previous SWAT of a brief PIL12 reported a similar difference in response rates between the brief PIL and control group, however this was not statistically significant. In our study, those returning an expression of interest in the SWAT intervention groups were more likely than those in the control group to not proceed at baseline due to no longer being interested or non-attendance at the appointment. It is possible that receipt of the pen and/or brief PIL may make return of the form more likely as a result of the hypothesised minor incentive, ease of form return or digestion of the study information – resulting in higher expression of interest rates – but that this does not then translate to randomisation when the requirements of participation are fully considered and understood. It is also possible that the brief PIL resulted in a small improvement in randomisation rate that we were not powered to detect, but that would nonetheless have been meaningful.
Although we did not observe evidence of statistically significantly higher randomisation rates, the SWAT interventions were low cost. It should be noted that improved recruitment rates may not be the only rationale for including similar interventions in future trials. For example, the provision of shorter, simplified recruitment materials may aid the representativeness of RCTs by reaching underserved populations.
Ideally, we would have collected demographic data on all participants who were sent an MSS3 invitation, in order to explore whether the SWAT interventions were of benefit to particular groups of participants - however this was not possible. Additionally, only limited data were collected on those returning an expression of interest form. We did not collect any qualitative data from participants with regards the SWAT interventions. This may have enabled us to explore the views of those receiving the interventions. If such data were collected, these would ideally include those who did not go on to participate in the host trial as well as those that did – although this would have been logistically challenging. The host MSS3 RCT was conducted both prior to and during the COVID-19 pandemic, with 59% of MSS3 invitations sent during the pandemic (post March 2020) – however, the number of participants in the SWAT arms were approximately equal pre-pandemic and during pandemic, so this is unlikely to have had an impact on the findings.
In conclusion, there was no significant evidence of effectiveness of the brief PIL intervention or the pen intervention on recruitment to the MSS3 study, but there was evidence of increased response rates to the initial invitation in the brief PIL group. The results of this SWAT should be combined with those from randomised SWATs testing the same interventions, in order to determine definitively whether they are an effective tool to improve recruitment rates.
White D: Funding acquisition, Methodology, Writing – original draft preparation, Writing – review & editing; Sutton L: Formal analysis, Visualisation, Writing – original draft preparation, Writing – review & editing; Mooney C: Funding acquisition, Methodology, Project Administration, Writing – review & editing; Dawson J: Funding acquisition, Methodology, Validation, Writing – review & editing; Burton C: Funding acquisition, supervision, writing – review & editing.
Per our info governance team, raw data are not able to be provided publicly due to the risk of identification. Readers and reviewers can apply for private access to the data via the following link: https://doi.org/10.15131/shef.data.22795418.
Data access (raw data file and dictionary) will be granted once users have provided a written proposal and consented to a data sharing agreement. Requests can be submitted to multiple.symptoms.study3@sheffield.ac.uk.
Aggregate data are available via the following:
figshare: MSS3 aggregate SWAT data, https://doi.org/10.15131/shef.data.23791731.v1. 16
This project contains the aggregate data file and dictionary.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The PROMETHEUS group: Catherine Arundel, Laura Clark, Adwoa Parker, Elizabeth Coleman, Catherine Hewitt, David Torgerson, Laura Doherty.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Orkin AM, Gill PJ, Ghersi D, Campbell L, et al.: Guidelines for Reporting Trial Protocols and Completed Trials Modified Due to the COVID-19 Pandemic and Other Extenuating Circumstances: The CONSERVE 2021 Statement.JAMA. 2021; 326 (3): 257-265 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Research methodology, trial design and reporting
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Patient information. SWATs.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 Sep 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)