Keywords
mucosal studies, north-south-south-north collaboration, technology transfer, capacity building in Africa, global health research
This article is included in the Global Public Health gateway.
mucosal studies, north-south-south-north collaboration, technology transfer, capacity building in Africa, global health research
It is important that there are global networks of excellence that can rapidly facilitate basic and translational research in response to new and emerging pathogens. The recent Covid-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has highlighted the need for a collaborative response with different groups working together and sharing advances in ‘real time’.
Countries in continental Africa feel a disproportionate effect of emerging and endemic infectious diseases. It is of vital importance that centers of excellence, in academic and scientific research, are developed in Africa, and the regional transfer of technology and knowledge supported. It is imperative to support and equip investigators, institutions, and associated stakeholders in the countries most affected by the diseases to allow them a leading role and active participation in research programs.
Over the last two decades there has been sustained, and increasing, investment in the development of basic and clinical research capacity in Africa.1 There is need to encourage more inter-institutional South-South technology transfer, within Africa, during collaborative clinical research. This type of support allows local scientists to initiate and drive cutting-edge research in sub-Saharan Africa (SSA).
The majority of South-South Collaborations (SSC) for technology transfer are large and intergovernmental, mainly involving Latin American nations, SSA, India, and China; with countries from SSA largely being recipients.2 In the health sector, most SSC within SSA have been supported by international charities or have occurred during emergency situations, as seen during the Ebola disease outbreak in 2014-2015.3 Mutual respect and benefit to all stakeholders is vital in all forms of the collaboration. Although there have been great achievements made through North-South collaborations, these may be accompanied with power imbalances.2,4 There exists ample capacity and underutilized talent and expertise in Africa that must be harnessed to foster SSC, and efforts towards this are underway, for example through the African Network for Drugs and Diagnostics Innovation (ANDI),5 the European and Developing Countries Clinical Trials Partnership (EDCTP)-supported networks of excellence (NoEs),6 the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE),1 and the International AIDS Vaccine Initiative (IAVI) network of independent partner research centers in SSA.7
IAVI, with support from the United States Agency for International Development (USAID) and other international partners, have over the last 20 years developed a network of independent partner research centers in SSA and India to facilitate the design and testing of vaccines against HIV-1. This ‘network’ of independent groups has leveraged this capacity to perform research and clinical trials into a number of other endemic diseases within the region, such as tuberculosis and Ebola, as well as catalyzing roll-out of HIV treatment and prevention for at-risk groups.8
Since the discovery of HIV-1, there has been a concerted effort to develop therapeutic and/or preventative interventions against the virus, including the development of an efficacious vaccine. While there have been a number of different approaches utilized in the design of vaccines, both in their composition and the vaccine regimen, the primary focus was initially based on assessing immune responses in peripheral blood to ‘triage’ vaccine candidates and determine potential vaccine efficacy in early phase studies. The vast majority of the global HIV-1 transmission occurs at the genital mucosa (cervico-vaginally, rectally, or through the penis foreskin, glans or urethra) during sexual intercourse.9 It is in these mucosal compartments where the initial infection gains an early foothold and where a preventative vaccine or microbicide is likely to be required to act. We demonstrated a strict genetic bottleneck in the number of viruses that are transmitted and establish infection.10 After several days, to weeks, the infecting virus replicates to such an extent that it passes through the draining lymph nodes11 into the peripheral circulation and other distal mucosal compartments, establishing infection and where it is likely to form a reservoir.
Many HIV vaccine and microbicide clinical trials are multisite and based in Africa and other low-middle income and resource-limited countries; which bear the burden of the HIV-1 pandemic and where HIV therapy, vaccines or other preventative interventions are most needed. In addition to the traditional approach of assessing systemic immune responses and depending on the vaccine mode of action, investigating the participant’s mucosal immune responses in clinical trials may be essential. Over the past few years, an increasing number of studies in SSA outside of South Africa have included mucosal sampling in such trials. Past studies have demonstrated the presence of HIV-relevant immune responses at mucosal sites.12 Moreover, the field of vaginal microbiome (VMB) has lately attracted significant interest due to the role of VMB in the transmission of genital infections, including HIV. Although VMB studies have been scarce in Africa, this in now changing especially with the recognized role of VMB in the transmission of HIV.13–16
Feasibility studies from Kenya showed that study participants accepted several mucosal sampling techniques from the genital and non-genital mucosal sites.12,17 For comparable data across studies and research centers, mucosal samples should be obtained in a standardized and reproducible manner. Unlike blood sampling, mucosal sampling methods have not been widely standardized, with many groups using different sampling equipment and methods, compromising data comparability and reproducibility across studies and study centers.
To foster inter-institutional South-South technology transfer, Kenya AIDS Vaccine Initiative-Institute of Clinical Research (KAVI-ICR) developed a mucosal training program to help standardize sampling and processing methods across the region and address the capacity gap in mucosal sampling techniques and immunology in Eastern Africa. While this was focused on developing and testing HIV-1 vaccines, the approaches developed can be used in a range of fields and diseases.
KAVI-ICR and the trainees’ research centers obtained local ethical approval for the respective research protocols. The research centers were KEMRI/Walter Reed Project (KEMRI/WRP)-Kericho Kenya, Center for Family Health Research (CFHR) Kigali Rwanda (previously known as Rwanda Zambia HIV Research Group-Projet San Francisco), UVRI-IAVI HIV Program Entebbe Uganda (UVRI-IAVI), KEMRI-Center of Geographical Medicine Research Coast (KEMRI-CGMRC) Kilifi Kenya, MRC/UVRI & LSHTM Uganda Research Unit (former MRC/UVRI Uganda Research Unit on AIDS Entebbe), Wits RHI, University of the Witwatersrand (WRHI) South Africa, Partners in Health Research and Development (PHRD), Kiambu Kenya, and Infectious Diseases Institute (IDI) Kasangati Uganda.
Kenyatta National Hospital-University of Nairobi Ethics and Research Committee granted approval for the studies at KAVI-ICR with the following ethical approval numbers: - P60/03/2008, P208/06/2010, P304/09/2010, P520/10/2012, and P353/05/2016, and P831/10/2019. KEMRI-CGMRC, KEMRI/WRP Kericho and PHRD received training at KAVI-ICR using two KAVI-ICR studies, approval numbers P60/03/2008 and P353/05/2016. Uganda Virus Research Institute Research and Ethics Committee granted approval for the studies at UVRI-IAVI (approval number GC/127/15/12/486), MRC/UVRI & LSHTM (approval number GC/127/12/07/33), and IDI (approval number GC/127/20/01/759). Rwanda National Ethics Committee approved the studies at CFHR (approval numbers 154/RNEC/2011, 373/RNEC/2012, and 820/RNEC/2019), and Wits Human Research Ethics Committee approved the study at WRHI (approval number 200101B). All participants in these studies gave informed consent prior to collection of the mucosal samples.
Development of the mucosal procedures: KAVI-ICR worked with IAVI to develop Standard Operating Procedures (SOPs) for the collection, transport, processing, storage and downstream immunological assays of gastrointestinal, oral, genital, ano-rectal and upper respiratory tract mucosal specimens as described elsewhere.12,17,18 Various sampling methods and devices were used. The procedures were applied into a range of studies to establish the feasibility of mucosal sampling and processing methods in an African setting,17,19 compared to the experience of this sample collection in Europe and the USA.20–22 Mucosal specimens included cells and secretions from the gastrointestinal, oral, genital, ano-rectal, and upper respiratory mucosal surfaces collected by various devices (Figure 1). All study participants provided informed consent and were given the opportunity to opt out of any mucosal sampling procedure. Reasons for refusal and other acceptability/tolerability data were collected. Depending on acceptability/tolerability and assay results, collection methods were ‘dropped’ or SOPs modified as needed. The resulting methods were subsequently applied into three Phase 1 HIV vaccine clinical trials at KAVI-ICR, all utilizing a common adenovirus 35 (Ad35) vector with varying vaccine regimens and prime/boost strategies, generating immunological data that were comparable across the trials.17,23
Mucosal training: After proof of concept studies at KAVI-ICR, a training program on mucosal specimen collection and processing was developed, in order to train other regional centers in the standardized collection and processing protocols.24 Several centers within IAVI partner research centers were targeted, but training was also provided in response to requests from centers initiating unrelated mucosal studies. In their request for training, each center specified the type of mucosal sampling technique they required training in. Training was either provided on-site at KAVI-ICR or at the trainees’ research centers based on the trainees’ preference, and under locally approved protocols allowing the collection of mucosal samples. Following the restrictions occasioned by the covid-19 pandemic in the year 2020, we introduced an online mucosal training option backed with video demonstrations.
For training that occurred on-site at the trainees’ research centers outside Kenya, it was first established that it was permissible for the trainers (medically certified in Kenya) to obtain clinical samples from study participants in that country. Prior to each training course the trainees’ center was advised on the required equipment and supplies, and KAVI-ICR/IAVI facilitated access to supplies and potential suppliers. As the consent process for mucosal sampling can be challenging, training was also provided in advance to ‘transfer’ the ‘soft’ skills acquired by KAVI-ICR staff during the development of their participant information sheets, informed consent documents and the consent process. Each center identified potential participants/volunteers for mucosal sampling in advance and provided preliminary information, including informing the volunteers of their involvement in the incumbent training and sample collection by visiting foreign trainers.
The studies used for the training were several and independent/unconnected, hence had varied inclusion/exclusion criteria for participants, recruitment methods and other study procedures. Common across the studies was that the participants were adults aged at least 18 years; and that the study procedures included mucosal sampling and processing. There were eight studies at KAVI-ICR; four of these were used to develop the mucosal procedures plus the training, the other four (including clinical trials) applied the developed mucosal procedures.
The training team consisted of at least one research physician, one research nurse, and a medical laboratory technologist. The training curriculum covered both clinical and laboratory procedures, and was designed to last for two and a half to five days depending on the number of trainees and the techniques to be covered.
SOPs training and introduction to consent for mucosal sampling: After familiarization with the local clinic and laboratory facilities, training commenced with didactic sessions during which KAVI-ICR staff shared their experience with mucosal studies and the lessons learnt, highlighting potential issues regarding obtaining informed consent for mucosal sampling. This was followed with a step-by-step discussion of the relevant SOPs, including a showcase of equipment.24 Trainees were then split into two groups for practical demonstrations, one of laboratory staff and the other of clinical staff.
Training in standardized mucosal sampling for clinicians: For the clinical demonstrations, the trainers and trainees were introduced to each potential volunteer and informed consent obtained prior to any mucosal sampling. KAVI-ICR clinicians (trainers) then performed a practical demonstration of each technique to the trainees. Subsequently, each trainee performed the techniques; initially assisted by the trainers, and then without assistance but under direct supervision. For the online training, the trainers used dummies instead of volunteers in the video demonstrations.
Samples were collected and processed as previously described.12,17 Briefly, Synthetic Absorptive Matrix (SAM) strips, swabs, merocel sponges, or the OriCol device were momentarily placed against respective mucosal surfaces (nasopharyngeal, oral, cervical, and rectal) to collect mucosal secretions. A cervical aspirator was inserted into the cervical canal and secretions aspirated. The Instead Softcup was placed in the vagina to collect secretions then removed, by a clinician or the participant depending on participant’s preference. The merocel sponges and swabs with collected secretions were each placed into a spin-X tube containing extraction buffer, and then placed on ‘wet’ ice until processing. The Instead Softcup with cervicovaginal secretions was placed in a 50ml falcon tube and kept on ‘wet’ ice. Saliva/oral fluid (transudate) was obtained by allowing a volunteer to drool through a drinking straw, while parotid gland secretions were collected using a salimetrics oral swab placed against the buccal mucosal surface of the parotid gland. Semen was collected into a sterile plastic container containing transport media. Endocervical and rectal mucosal cells were collected by placing a Digene cytobrush on the endocervical or rectal mucosa then rotating it against the mucosal surface to obtain mucosal cells. Rectal biopsies were collected by pinching off the rectal mucosa using Sarrratt rectal pinch biopsy forceps. Cytobrushes with collected cells and rectal biopsies were placed in 15ml falcon tubes containing transport media at 40C until processing. All samples were transported to the laboratory and processed within two hours of collection.
Access to cervicovaginal and rectal mucosal surfaces was achieved using a sterile and disposable, vaginal speculum and a rectal proctoscope respectively.
Mucosal sample processing and storage training for laboratory personnel: Training covered processing and storage of all sample types, and several routinely used downstream assays and analyses as described previously.12,18 Mucosal secretions were eluted from the swab/sponge by centrifugation then analyzed for antibodies using Enzyme Linked Immunosorbent Assays (ELISA). Cellular samples harvested from the Digene cytobrushes, and where applicable single cell suspensions from Collagenase II digested biopsies, were stimulated and analyzed using standardized multiparametric flow cytometry with intracellular cytokine staining. Flow cytometry data were analyzed using Flow-Jo Software (version 10.0.8; Tree Star, Ashland, Oregon) after acquisition.18 Flow-Jo was provided to colleagues in Africa through the Flow-Jo Africa program which provides free licenses for researchers on the entire African continent who are enrolled with an African University and permanently located in Africa. Subsequent to training, there was follow-up with the regional centers to support their establishment of the assays, including continued sharing of SOPs. Whenever required, we arranged regular contacts via email or online calls for discussion of experimental design and troubleshooting where necessary.
Development of the mucosal procedures: Clinical and laboratory SOPs were developed for the collection, processing and storage of:
- Rectal and cervical secretions using Merocel sponge;
- Cervicovaginal secretions using the Instead Softcup;
- Cervical secretions using the cervical aspirator;
- Seminal fluid by self-masturbation;
- Parotid gland secretions using the Salimetrics Oral Swab;
- Whole oral fluid (transudate) via a drinking straw;
- Nasal and nasopharyngeal secretions by the nasal flocked swab;
- Nasal secretions using the SAM strip;
- Rectal secretions using the OriCol rectal device;
- Rectal and cervical mucosal cells using the Digene cytobrush;
- Rectal mucosal cells using Sarrratt pinch forceps; and
- Gut mucosal cells using gut biopsy forceps.
These methodologies were subsequently successfully applied in three epidemiological mucosal studies at KAVI-ICR in the years 2009 to 2013 and thereafter in four collaborative Phase 1 HIV vaccine clinical trials, a Varicella Zoster vaccine trial, basic science studies, and most recently an HIV broadly neutralizing antibodies clinical trial. (Table 1)
Mucosal training: We, since the year 2011, have trained eight research centers from Uganda, Rwanda, South Africa and Kenya. The centers were KEMRI/WRP-Kericho Kenya, CFHR Kigali Rwanda, KEMRI-CGMRC Kilifi Kenya, MRC/UVRI & LSHTM Uganda, WRHI South Africa, PHRD Kiambu Kenya, and IDI Kasangati Uganda. All the centers, except WRHI, received training on the sampling of cervicovaginal mucosal secretions using the merocel sponge and/or Instead Softcup, while one center did not receive training on sampling of rectal secretions. Additionally, CFHR Rwanda received training on the collection of oral and upper respiratory secretions; KEMRI/WRP-Kericho and KEMRI-CGMRC Kilifi received training on rectal and cervical cell sampling using the cytobrush; KEMRI-CGMRC Kilifi further received training on rectal biopsy sampling using pinch forceps. CFHR, IDI, WRHI, PHRD, and UVRI-IAVI received training on rectal sampling using the OriCol rectal device in the year 2021. CFHR, MRC/UVRI & LSHTM, UVRI-IAVI, KEMRI/WRP, KEMRI-CGMRC, and PHRD had physical training; all three centers from Kenya received training at KAVI-ICR, while three centers from outside Kenya received training on-site at the trainees’ research centers. In the year 2021 all (four) centers from outside Kenya (CHFR, UVRI-IAVI, IDI, and WRHI) received training online. (Table 2)
A total of 108 research staffs received training with CFHR having the highest number of trainees (n=34, 32%). The trainees’ cadres included physicians/clinicians (28%), nurses (33%) and laboratory technologists (39%). (Figure 2)
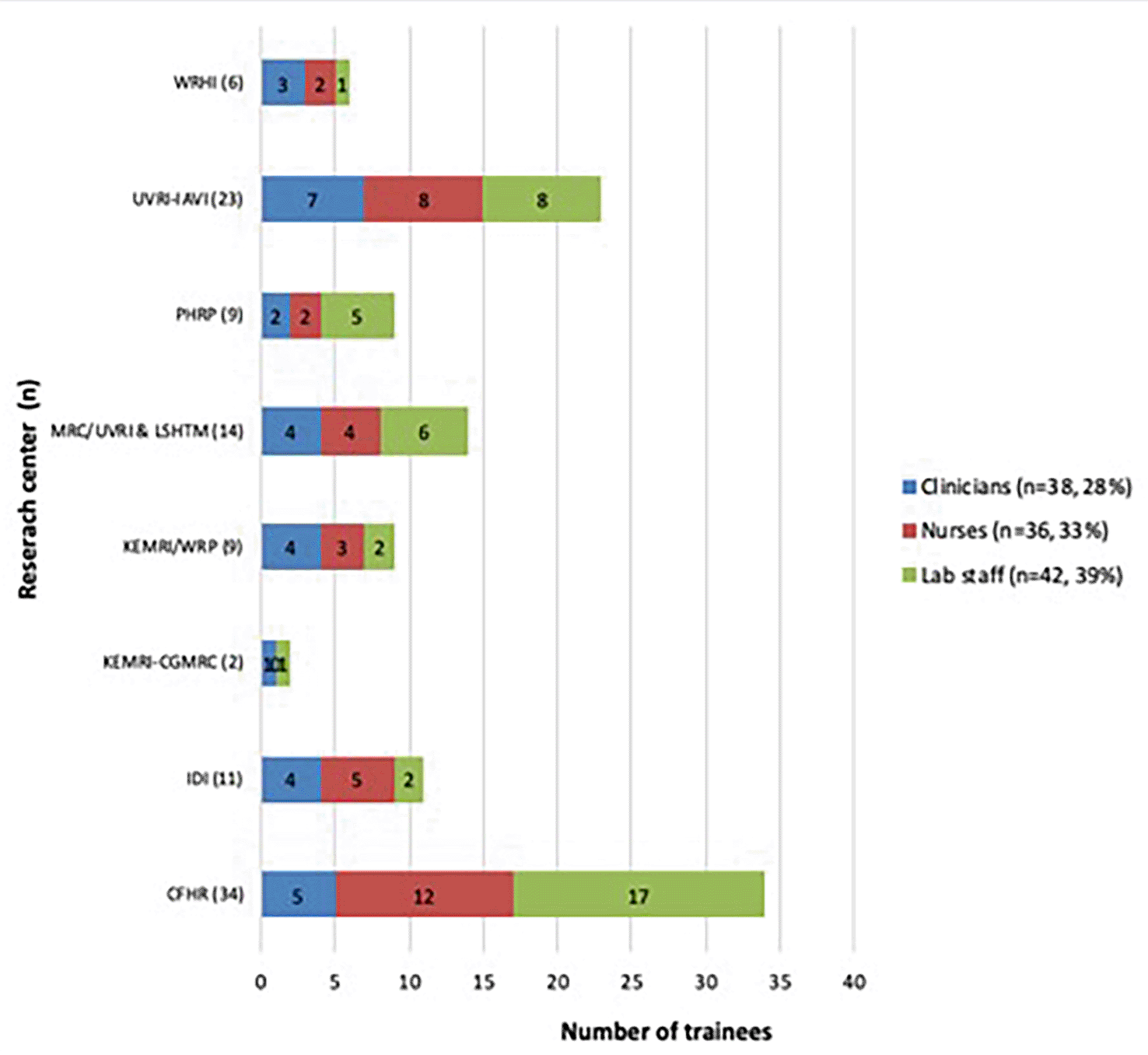
CFHR - Center for Family Health Research, Kigali, Rwanda; IDI - Infectious Diseases Institute (IDI) Kasangati, Uganda; KEMRI/WRP - KEMRI/Walter Reed Project, Kericho, Kenya; KEMRI-CGMRC - KEMRI-Center of Geographical Medicine Research Coast, Kilifi Kenya; MRC/UVRI & LSHTM - MRC/UVRI & LSHTM Uganda Research Unit, Entebbe, Uganda; PHRD - Partners in Health Research and Development, Kiambu, Kenya; UVRI-IAVI - UVRI-IAVI HIV Program Entebbe Uganda; WRHI - Wits Reproductive Health and HIV Institute, South Africa.
All the centers that received training have since applied the skills learnt into a range of mucosal immunological studies. CFHR, Rwanda applied the mucosal skills in a collaborative multi-country observational study (with St. Mary’s Hospital, London, UK and KAVI-ICR),12 and during a clinical trial in collaboration with KAVI-ICR, Kenya, and St. Stephens AIDS Trust, London, UK12,17,23; the resulting mucosal immunological assay results were comparable across the participating sites. CFHR Rwanda, IDI Uganda, PHRD Kenya, UVRI-IAVI Uganda, WRHI South Africa, and KAVI-ICR Kenya are all applying the mucosal techniques in an ongoing multisite clinical trial; in this clinical trial KAVI-ICR is the regional center for the mucosal assays.25
Furthermore, laboratory personnel from three research centers in India were also trained in the laboratory methods developed by KAVI-ICR. These included staff at the National AIDS Research Institute Pune, YRG Care Chennai, and National Institute for Research in TB, Chennai; the training provided included mucosal samples processing, storage and immunological assays.
We present a paper describing how a center of excellence at KAVI-ICR, Nairobi, Kenya, supported by IAVI, USAID and multiple international donors has developed inter-institutional South-South collaborations in Africa through facilitating technology transfer and the sharing of experiences and best practices in mucosal immunological studies. KAVI-ICR spearheaded successful mucosal studies and the transfer of technology to other SSA research centers, making it possible to standardize mucosal sampling and processing across the research centers, obtaining analogous samples that yield comparable data.12,23
The development of mucosal studies led to an increase in basic research, and resulted in the development of clinical trials in which assessment of mucosal responses is embedded within the clinical trial protocol.17,23,25,26 Consequently, in collaboration with IAVI’s USAID-funded mucosal immunology program, the IAVI extended network of clinical research centers developed a ‘blanket’ protocol to allow the collection of peripheral and limited mucosal samples in order to support the transfer of knowledge and training in these standardized methods, and to allow rapid sample collection for collaborative pilot studies to generate preliminary data.27 While focused on studies that support the development of an HIV-1 vaccine, these methods are available to new collaborators to kick-start collaborative studies and utilize the capacity developed in these state-of-the-art clinical research centers. Indeed, these methods have already been applied in a varicella zoster vaccine study at KAVI-ICR in collaboration with University of Toronto.26
Importantly the nature of mucosal immunology and its requirement for fresh assays to be performed on site has facilitated the engagement of local staff at the research centers and the development of increased staff and infrastructure capacity on site. This has allowed local scientists to drive aspects of research to develop their own careers, actively engage with the project teams, and develop lasting collaborations and mentorship with international partners.
For example, researchers at KAVI-ICR are now employing the Widefield microscopy technology to studies assessing the replication of HIV transmitted/founder viruses in cervical and rectal tissues ex vivo, mucus mobility studies, and human tissues studies for HIV reservoirs.28
Although our program was initially designed for in-person delivery we were, out of necessity, able to successfully execute an online training as well. The four sites that were trained online have subsequently successfully applied the mucosal techniques in a multisite and multi-country clinical trial,25 from which we anticipate a yield of comparable data. One of the advantages of online training is the savings on travel and accommodation costs; such savings should be desirable especially in the SSA region where resources are limited. Therefore, online options for SSC in capacity building need to be purposely promoted.29 For this the SSA institutions can seek support from organizations such as the South-South Galaxy which fosters online SSC through advisory services, capacity advancement, linkage to experts, among others.30
Another addition to our original design of the program was the extension of the technology transfer program outside the African continent, supporting training of partners in India. We have thus been able to progress from purely transferring methods in a North-South manner to supporting North-South-South-North collaborations for technology transfer.
Our program did not have a formal monitoring and evaluation plan; we instead relied on random follow-up with the trained centers for troubleshooting and to support establishment of assays. However, we only received a few and minor post-training queries and consultations, and there were no requests from the research sites for follow-up repeat/refresher training. The only additional training requests were for new techniques not covered previously e.g. use of the OriCol device for the collection of rectal secretions. Hence, we believe that the trainings were effective. Nevertheless, we shall in future include a quality control and evaluation plan in the program. A second limitation was that we did not advertise our courses widely and instead relied on the sites soliciting for the training; this may have limited our reach. Despite this we trained several centers across four countries. Besides, we ultimately were able to successfully implement a tailored training (physical and online) from which we can henceforth confidently expand our reach via open calls for trainees.
We successfully established and implemented a South-South mucosal training program in SSA. Our venture for sharing of experiences and best practices, also yielded collaborative research projects across the participating sites. This initiative has now expanded into a state-of-the-art mucosal immunology program, through which KAVI-ICR has expanded its research portfolio.
Lessons from our mucosal capacity building program can be extrapolated to SSC for capacity strengthening and technology transfer in other areas of the clinical research processes spectrum - from research conceptualization, grant writing, research inception and conduct, up to dissemination of research findings.
SSC in research should be encouraged; through this, robust local networks of excellence for expanded collaborative research are realizable. Additionally, pulling together in the principle of horizontality is beneficial for balanced and triangular cooperation in research. Through such initiatives, supported by international donors, the development of regional capacity and expertise is possible. The established expertise can be leveraged when needed, and also builds the capability for African scientists to engage at an international level, actively participating in driving internationally relevant research.
Establishment and promotion of research capacity and expertise in Sub-Saharan Africa is essential, for leveraging when needed. This paper describes a regional South-South technology transfer initiative in clinical research, whose advancements are facilitating advanced research and new collaborations in Sub-Saharan Africa. South-South technology transfer in research should be promoted; it builds the capability of African scientists to actively participate in driving internationally relevant research and provides networks for rapid local response to novel emerging pathogens.
Flow-Jo was provided to colleagues in Africa through the FlowJo Africa program which provides free licenses for researchers on the entire African continent who are enrolled with an African University and permanently located in Africa. Flow cytometry data can also be analysed using the software provided with individual Flow cytometry machines or using freely available software such as Floreada.io.
Figshare: Extended data - Mucosal sample collection, processing and technical assay methods used in the establishment and implementation of a regional mucosal training program to facilitate multi-center collaboration in research in Eastern Africa.
https://doi.org/10.6084/m9.figshare.24116082 (Omosa-Manyonyi GS et al., 2023).
This project contains the following extended data:
• Data file 1. Presentation: Mucosal training - Introduction.pdf
• Data file 2. Presentation: Mucosal training - Oricol device and Softcup mucosal sampling & processing.pdf
• Data file 3. SOP 33 version 4.0 – Collection of Rectal and Cervical Cytobrush and Merocel Specimens and Rectal Biopsy.pdf
• Data file 4. SOP 34 version 2.0 - Collection of Semen Sample.pdf
• Data file 5. SOP 0075 – Collection, transport, processing and storage of oral fluid (passive drool saliva).pdf
• Data file 6. SOP 0076 – Collection, transport, processing and storage of nasal samples using Flocked swabs.pdf
• Data file 7. SOP 0078 – Collection, transportation, processing and storage of Vaginal samples using an Instead softcup.pdf
• Data file 8. SOP 0080 – Collection, transport, processing and storage of cervico-vaginal samples using a Merocel sponge.pdf
• Data file 9. SOP CL 49 - Procedure for processing Semen.pdf
• Data file 10. SOP CL 55 - Collection of passive Drool Saliva version 1.1.pdf
• Data file 11. SOP CL 56 - Collection of Saliva direct from the Parotid gland Using the Salimterics Oral Swab version 1.1.pdf
• Data file 12. SOP CL 60 version 1.0 - Nasal Sample Collection (SAM).pdf
• Data file 13. SOP IMM 024 - Collection transport processing and storage rectal secretions using an OriCol sampling device V1.0_250521.pdf
• Data file 14. SOP SLM 0016 - Isolation of human mucosal cells from colonic rectal or duodenal tissue.pdf
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We are grateful to all the research participants who volunteered for the mucosal studies. We also wish to thank all the research centers that participated in the mucosal training i.e. KEMRI-CGMRC, KEMRI/WRP Kericho, UVRI-IAVI, MRC/UVRI & LSHTM, CFHR, IDI, WRHI, and PHRD.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: immune surveillance, mucosal immunology, infectious diseases (mainly respiratory)
At the request of the author(s), this article is no longer under peer review.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 28 Sep 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)