Keywords
Pancreatic ductal adenocarcinoma, neoadjuvant chemotherapy, pathology, surgery, prognosis
Pancreatic ductal adenocarcinoma, neoadjuvant chemotherapy, pathology, surgery, prognosis
Pancreatic ductal adenocarcinoma (PDAC) is aggressive cancer with poor prognosis.1 Surgery represents the only curative treatment. In the last decade, the possibility of surgical resection of borderline and locally advanced PDAC (BR/LA-PDAC) was increased by the implementation of preoperative chemotherapy.2 Indeed, pathological complete response (pCR) in PDAC is observed in about 3% to 33% of cases.3,4 In addition, previous single-institutional studies have demonstrated significant improvements in OS among patients who experience pCR.5 For this reason, pCR is considered a positive prognostic factor as reported by several studies.6–8 This case report aims to highlight the necessity to develop preoperative chemotherapy to improve oncological outcomes.
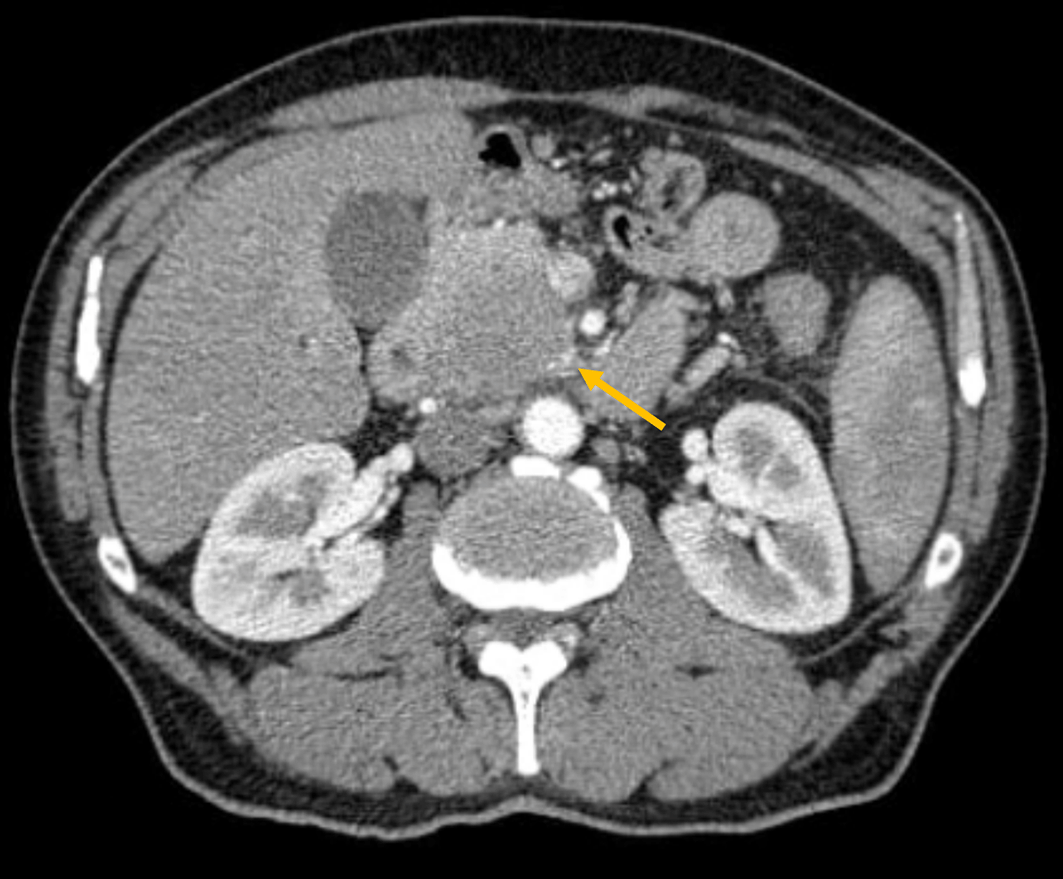
A 60-year-old male, with a past medical history of type 2 Diabetes Mellitus, presented for two months’ history of obstructive jaundice, vague abdominal pain, and weight loss. Biological data objective an elevated serum total bilirubin at 300 μmol/L, direct bilirubin at 220 μmol/l, alkaline phosphatase at 300 IU/L, gamma-glutamyl transferase at 194 U/L. A multiphasic thin-slice Computed Tomography, shown in Figure 1, diagnosed a three-centimeter pancreatic head Tumor. Pancreatic Cancer Staging was T2 N1 M0 borderline resectable pancreatic cancer according to NCCN. The endoscopic ultrasound found a three-centimeter pancreatic head hypoechoic heterogeneous solid lesion with irregular contours. This mass has close contact with the superior mesenteric vein (SMV) (<180°). The diagnosis of ductal adenocarcinoma was confirmed by an endoscopically guided biopsy. In the liver MRI with diffusion-weighted sequences, there was no metastasis. Each key stage in the treatment is discussed during a multidisciplinary consultation meeting and then proposed to the patient. To this end, the patient had Systemic chemotherapy with six injections of FOLFIRINOX without radiotherapy. The radiological re-staging, shown in Figure 2, revealed a cancer down-sizing of 2 cm and tumor became resectable. So, the Whipple procedure was performed. The postoperative follow up was uneventful and the patient was discharged on day 13. The pathological examination of the specimen, shown in Figure 3, concluded to pCR. The twelve lymph nodes evaluated were negative. Following surgery, adjuvant chemotherapy FOLFIRINOX was given. After three years of follow-up, there is no evidence of recurrence.
Our case reflects the importance of neoadjuvant therapy as part of the multidisciplinary approach of pancreatic ductal adenocarcinoma. Pancreatic cancer is one of the most aggressive malignancies. Surgery remains the main curative therapy. Despite a more aggressive technique that aims to increase R0 resection rate; the long-term prognosis after surgery remains poor with a five-year survival rate of 20% in the best series.4 This raises the question of whatever pancreatic cancer should be considered as a systematic disease and the necessity of neoadjuvant therapy even in resectable cases. Preoperative chemotherapy or chemo radiotherapy has many theoretical advantages. It induces a down staging, treats micro metastatic lesions, and increases the rate of R0 resection margin. In addition, associating radiotherapy to chemotherapy may enhance the pathological response. In fact, higher rates of complete response were reported after associated radiotherapy.9,10
Although, its benefit on borderline and locally advanced cancer was confirmed; its role in inducing pCR remains unclear. Moreover, regarding the absence of a standard definition and the rarity of such an event; there is a lack of evidence supporting the positive impact of pCR on long term outcomes. Two large cohorts published recently reported a greater overall survival and disease-free survival for patients presenting pCR in the operative specimen.11,12 Yamada et al.13 in a retrospective trial including 594 PDAC reports an encouraging five-year overall survival in pCR even with residual intraductal carcinoma component. The residual carcinoma in situ is due to the higher resistance to of intraductal component tumor cells to preoperative treatment.14 Cloyd et al. reported the data of 7902 patients who received NT before pancreatic resection.5 24.3 patients [3.1%] experienced pCR. OS was found to be significantly better in the pCR group [76.6 versus 26 months, p<0.001]. On multivariate analysis, the use of multi-agent neo-adjuvant chemotherapy, duration of NT and pCR were associated with improved OS. Predictive factors of pCR were investigated among these patients. Only longer NT and the use of radiation were found to be independent factors. In the same way, several reports investigated the accuracy of imaging modalities in predicting pathological response after neoadjuvant therapy. Barreto et al.15 analyzed data from fifteen studies and 995 patients with borderline resectable or locally advanced ductal pancreatic carcinoma whose underwent neoadjuvant therapy. A total of 60% of patients underwent surgery with R0 rate of 88%. Down-sizing, in CT-scan before surgery, was observed only in 20% of patients. Such results could be explained by the inflammation induced by neoadjuvant therapy which may mimic a solid tumor. Mellon et al.10 in a cohort of 81 patients, reported a significant correlation between post neoadjuvant therapy SUV max and TRG. Patients with a 100% reduction in SUV max had a complete pathological response. CA-19-9 was also investigated as a biomarker that predicts response to neoadjuvant therapy. Mellon et al.10 reported that CA-19-9 level before surgery was the most strongly correlated to pCR response. Lee et al.16 reported the same results. CA-19-9 level after neoadjuvant therapy was significantly different between pCR group and non-pCR group. However, the use of this biomarker may present some limitation, especially in presence of biliary obstruction or in patients who present with a normal CA-19-9 level. Future work should identify additional markers to predict pCR.
According to NCCN guidelines [version 1.2020], additional therapy is recommended after surgery for ductal pancreatic adenocarcinoma. Its role in patients who experienced pCR remains, however, a divisive topic. Kourie et al.17 in a French multicentric cohort, reported the characteristic and the outcomes of 29 patients with pathologic complete response after preoperative treatment. Only eight patients received postoperative chemotherapy. No difference was found in DFS or OS when comparing the 8 patients to those who did not receive adjuvant therapy. These findings were however infirmed in a second cohort published by Lee et al.16 Among the nine patients who experienced pCR, four presented tumor recurrence [2 in the liver and 2 in the peritoneum] within 1 year, raising the question of the necessity of complementary treatment even in those patients.
The pathological complete response following neoadjuvant therapy is a rare event which rarely exceeds 6%. Some limited series suggested a positive impact of pCR on DFS and OS,6–8 but this role had to be confirmed. Routine radiological exams are currently limited to stage the pathological response of PDAC following neoadjuvant therapy. CA-19-9 may be a strong predictor of pCR in patients with pancreatic cancer, but its use presents some limitation.
Written informed consent obtained from the patient for publication of this case report and any accompanying images.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: CARE checklist for ‘Complete pathological response after chemotherapy for borderline pancreatic cancer’, DOI: 10.6084/m9.figshare.24069210.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
References
1. Versteijne E, van Dam JL, Suker M, Janssen QP, et al.: Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial.J Clin Oncol. 2022; 40 (11): 1220-1230 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical outcomes pancreas cancer
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 05 Oct 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)